This volume will be potentially useful both for clinicians who are dealing with infections associated with biofilms and for investigators who are interested in this subject. Each of the chapters is self-contained to a designated topic, thereby allowing for their reading independent of the rest of the book. This is particularly true of the chapters in section 1, which provide an excellent background summary of biofilms and their significance. However, by reading the book from beginning to end, one can get a good sense of the current level of understanding of and research in the role of biofilms in Clinical Infectious Diseases.
The level of presentation of material is such that even a novice to the topic can gain insight into and an initial understanding of how bacteria live in and interact with biofilms in pathogenic environments. However, many of the chapters are detailed enough to provide useful material for clinicians and investigators who are more seasoned in this area.
Taken together, the book has the potential to serve as an excellent starting point for potential investigators in this subject and as good reference work for established investigators. There are several limitations of this book. Because each of the chapters appears to have been written to have the potential to stand alone, there is a fair amount of redundancy of background material on biofilms. Although this may serve as a learning tool for the novice, it might also serve as a nuisance to some readers who will tire of reading the same thing over and over again.
At times, the level of detail in the chapters that describe the role of biofilms for specific pathogens or specific antimicrobial agents is potentially too extensive. However, the chapters on treatment protocols for specific infections e. Finally, a thorough reading of this book will not identify any clearly effective strategies aimed at the long-term prevention of biofilm-related infections.
Although this likely reflects the absence of such prophylactic options, it was nevertheless disappointing. In summary, this book is recommended for graduate students and infectious diseases trainees, as well as for established scientists and clinician investigators who are interested in the subject of biofilm-related infections. It will also serve as a useful reference book for infectious diseases divisional libraries, where individual chapters could clearly serve as useful introductions to and reviews of this topic.
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Close mobile search navigation Article navigation. Acknowledgments Potential conflicts of interest.
Add comment Close comment form modal. Moreover, a deletion mutant accumulated more tobramycin than the wild type, suggesting that the identified locus could code for an efflux pump. The last identified gene, tssC1 , was shown to be an essential component of the type VI secretion T6S system potentially involved in cell-to-cell interactions More recently, three other loci, i.
Several studies performed in P. From the sputa of CF patients, highly tolerant P. Strikingly, deletions in the mexXY locus restored wild-type resistance but did not affect antibiotic tolerance, suggesting that MexXY-OprM plays only a marginal role in biofilm recalcitrance When inactivated, biofilm bacteria are more susceptible to hydrogen peroxide and antibiotics of different classes, including tobramycin and norfloxacin.
On the other hand, brlR overexpression increased P. High-thoughput screening of E. It was demonstrated that rapA not only regulates yhcQ , a gene encoding a putative multidrug resistance MDR pump, but also yeeZ , a gene suspected to be involved in ECM production Thus, a dual rapA -mediated action was proposed, with efflux through a pump and reduced penetration through an increase in polysaccharide production The hypothesis of biofilm-induced E. However, these pumps play a role mostly at low antibiotic concentrations, and, to date, the involvement of biofilm-specific efflux pumps in biofilm recalcitrance has remained controversial 14 , 70 , 73 , While the above-mentioned mechanisms play an important role in the inability of antibiotics to fully eradicate biofilm bacteria, they cannot fully explain biofilm recalcitrance.
This is particularly clear in the case of fluoroquinolones: The presence of persisters in the bacterial population has been known since the origin of the antibiotic era; indeed, Joseph Bigger identified them in a population of S. He then showed that this subpopulation of bacterial cells resumed growth after the end of antibiotic exposure and that they were not resistant mutants, since they exhibited the same survival pattern following another exposure to antibiotics.
Retrospectively, he described most of what constitutes our current knowledge of persisters Fig. Main phenotypic characteristics of persister cells. A Persisters red bacteria are present under planktonic and biofilm conditions and account for only a small subset of the whole population 0. B Persisters are not resistant mutants. After treatment of a bacterial population with a bactericidal antibiotic, all nonpersister cells die, giving a biphasic survival curve.
After a rapid decrease, surviving cell fractions reach a plateau corresponding to persisters red curve. After antibiotic removal and addition of rich medium, persisters resume growth. The population obtained displays a susceptible phenotype toward the antibiotic blue curve. If a resistant mutant were present, it would be able to grow in the presence of the antibiotic dotted line.
Panel B was inspired by previous reports 13 , 31 , At the population level, the presence of persisters can be viewed as an insurance strategy In case of intense stress for the community, persisters may survive and permit the survival of the community. It has also been proposed that persisters might be bacteria that escape antibiotic-induced programmed cell death PCD.
In that case, the antibiotic is able to interact with its target, thereby leading to growth inhibition; on the other hand, bacteria do not die because of inactivation of PCD Analysis of survival curves of antibiotic-treated biofilms suggested the presence of a subpopulation of tolerant bacteria surviving bactericidal antibiotics despite increased concentrations and times of exposure, leading to the hypothesis that persisters may play a part in biofilm recalcitrance toward antibiotics Indeed, when exposed to increasing concentrations of fluoroquinolones active against nondividing cells and without any diffusion impairment , biofilm bacteria are killed until reaching a survival plateau, thereby creating a biphasic curve such as those seen under planktonic conditions Fig.
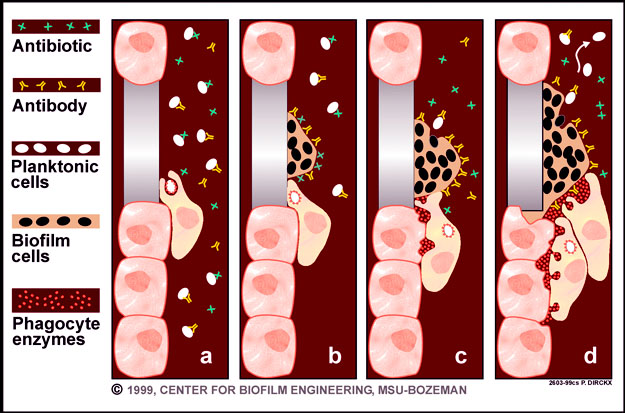
These tolerant bacteria are mostly persisters, and many in vitro , in vivo , and clinical studies support the idea that they are responsible for most of the antibiotic recalcitrance of biofilms. Several in vitro and in vivo studies demonstrated the presence of highly tolerant bacterial persisters in biofilms formed by Gram-positive and Gram-negative pathogens 86 , — Clinical demonstration of the presence of persisters can be inferred from the risk of infection recurrence during biofilm-related infections. Thus, the currently proposed model to explain biofilm recalcitrance toward antibiotics relies mainly on the presence of persister cells 13 , For antibiotics such as fluoroquinolones, which freely diffuse through the matrix and kill nondividing bacteria, impaired antibiotic diffusion, drug indifference, and specific genetic mechanisms play minor roles in biofilm recalcitrance.
Conversely, persisters are able to survive antibiotic-mediated bacterial cell death induced by any bactericidal antibiotics. Furthermore, persisters inside the biofilm matrix escape the effect of the host immune system. Once antibiotic treatment is withdrawn, persisters hiding in the matrix can resume growth, repopulate the biofilm, and cause infection recurrence. Because persisters are isogenic and present prior to the introduction of antibiotics, they are now believed to appear through a phenotypic switch Several factors and mechanisms have been described as playing important roles in the occurrence of this switch.
Most studies on the molecular mechanisms involved in persister formation were conducted with planktonic rather than biofilm bacteria. It is now believed that the presence of persisters is related to both passive and active mechanisms, environmental factors, and stochastic gene expression. Dormancy can be defined as a state of low metabolic activity during which bacteria do not proliferate without a resuscitation phase Therefore, truly dormant cells do not display metabolic activity.
Different lines of argument suggest a link between dormancy and persistence. By use of microfluid devices, E.
Furthermore, using an unstable fluorescent reporter gene associated with a ribosomal promoter rrnB P1, which controls expression of rrnB genes expressed at high levels during growth , it was shown that a weakly fluorescent population i. Even when enrichment is significant, it is important that not all dormant bacteria are persisters; conversely, all persisters do not necessarily correspond to dormant cells Therefore, it is likely that passive dormancy per se is not entirely responsible for the persister phenotype A recent study confirmed these findings by using flow cytometry sorting of E.
The authors showed that bacteria that grow rapidly prior to antibiotic exposure can give rise to persisters, whereas low metabolic activity or a low growth rate only increases the odds of entry into persistence Expression of toxin-antitoxin TA modules often leads to a shutdown of bacterial cellular processes. Although the molecular nature of TA modules varies, from protein to RNA molecules, the toxin is usually a stable component that inhibits major cellular functions, such as translation and replication 96 , To keep a toxin in check, degradable antitoxin antagonizes the effect of the toxin through formation of an inactive complex.
In the case of a TA module carried by a plasmid, after cell division, newly formed daughter cells die unless receiving the plasmid, as the antitoxin will be degraded through proteolysis, allowing the toxin to exert its deleterious effects in plasmid-free bacteria. Since toxins halt growth and thus reduce the activity of the antibiotic target, they appear to be attractive effectors of the switch to the persister state , This hipA7 allele is associated with two point mutations resulting in a gain of function.
As overexpression of HipA is toxic and leads to the arrest of cell division, it has been proposed that the locus carries a toxin-antitoxin module The deletion of hipB is lethal because of HipA toxicity, suggesting that HipB is the repressor of the operon Note that deletion of the complete hip locus has no effect on persister frequency in exponentially growing bacteria, possibly because of TA module redundancy 94 , Another explanation is that the HipBA module contributes to the persister switch only in cases of slow growth stationary-phase cultures HipA was first thought to phosphorylate the translation factor EF-Tu, leading to persistence via cell stasis However, it was recently shown that HipA more likely inhibits glutamyl-tRNA synthetase GltX through phosphorylation and thus triggers the synthesis of ppGpp see below Using an hipA7 E.
The study confirmed that overexpression of relE led to growth inhibition and increased the level of persisters. Note that, in E. Deletion of the hipBA locus leads to a decrease in the level of persisters in stationary-phase culture. Conversely, deletion of the other identified TA modules had no effect on the level of persisters in stationary-phase culture, suggesting a probable redundancy Redundancy was later confirmed when Maisonneuve et al. The same group used a flow cytometer to sort E. Overexpression of ygiU led to growth inhibition and also increased the levels of tolerance toward ofloxacin and cefotaxime but not tobramycin.
Recently, a new type of TA module, type V, was associated with persistence in E. Interestingly, the authors also identified a possible interaction between GhoST and MqsR, a toxin that, upon inactivation, decreases formation of persister cells Thus, when MqsR is induced, ghoT is still expressed and can contribute to persistence. Indeed, deletion of ghoT decreases MqsR-mediated persistence, and mild production of the GhoT toxin leads to persistence upon ampicillin treatment.
Lastly, expression of the F-plasmid-based CcdAB TA system increases the persister level and could constitute a transmissible persistence factor see below Therefore, it appears that various TA modules have different and cumulative effects under different conditions, suggesting a certain level of redundancy. Another way to link TA modules and persister genesis would be through degradation of the unstable antitoxin, which ultimately would lead to activation of the toxin. In this regard, recent studies on the effects of the stringent reponse and Lon protease led to establishment of new connections between starvation and persistence.
When a bacterial culture is kept in exponential phase with continuous dilution and constant medium renewal, persisters disappear Conversely, at late stationary phase, the percentage of persisters increases and reaches a maximum, suggesting the importance of starvation in the genesis of persisters This may be explained by indole production during stationary phase and nutrient limitation, leading to increased levels of E.
Note that it was previously shown that indole production was increased in response to oxidative stress and antimicrobial exposure, through upregulation of the tnaA gene, which is responsible for indole synthesis Because the ppGpp-mediated stringent response is induced in cases of nutrient limitation, it was suspected of playing a role in the phenotypic switch of persisters 20 years ago. In , ppGpp overexpression in E.
Strategies for combating bacterial biofilm infections
Thus far, two major connections between the stringent response and persistence have been described: Upon amino acid starvation, induction of the stringent response upregulates catalase activity The demonstration of a link between the stringent response and oxidative stress defense is interesting, as ROS have been proposed to explain antibiotic-induced bacterial cell death , — This subject remains a matter of intense debate and controversy, as other scientists recently published conflicting results that contradict this theory , — However, it might be envisaged that because of the stringent response, bacterial persisters will be less damaged by ROS and thus exhibit tolerance Fig.
For instance, it was shown that in P. Main factors involved in generation of persisters. The stringent response A and the SOS response B are now considered pivotal in the generation of persisters. C Connection between stochasticity and persister genesis. In exponential-phase cultures, due to stochasticity, only a few bacteria reach the required threshold of a toxic molecule that is necessary to switch to the persister state in red.
Due to the factors described in panels A and B, there is an increased level of molecules inducing persistence; thus, more bacteria reach the threshold and become persisters. Note that most of these studies were conducted with planktonic bacteria. Panel C was inspired by a previous report In the last 10 years, major studies have increased our understanding of the connection between the stringent reponse, TA modules, and persistence, and two main models have been proposed.
For a comprehensive overview of this question, see reference The first model proposes that Lon protease plays a central role. The stringent response alarmone p ppGpp inhibits exopolyphosphatase, thus increasing the level of inorganic polyphosphate and ultimately inducing Lon protease activity , It has been demonstrated that the Lon protease inactivates type II antitoxin molecules, including HipB , The degradation of the related unstable antitoxin by Lon leads to an increased ratio of toxin to antitoxin, translation and replication arrest, and thus tolerance Fig.
The same group demonstrated that p ppGpp stochastically triggers the activation of TA modules and thus controls the frequency of persisters Interestingly, a reverse model was proposed in for E. It was suggested that free Hip toxin increases the level of ppGpp, thereby leading to altered gene expression and thus priming cells for the phenotypic switch More recently, overexpression of HipA was shown to trigger growth arrest by inducing RelA-mediated synthesis of ppGpp These conflicting results were explained in a recent study in which the authors demonstrated that free HipA inactivates GltX the glutamyl-tRNA synthetase through phosphorylation.
This event leads to the accumulation of uncharged tRNA Glu in the cell, which induces RelA-mediated activation of the stringent response Ultimately, the level of ppGpp increases, leading to growth arrest and persister formation Fig. The second model to explain the connection between the stringent reponse, TA modules, and persistence suggests that the stringent response inhibits DNA supercoiling.
Furthermore, the authors demonstrated that ppGpp-SpoT acted as a TA module on its own, with the following lines of argument: In this ppGpp-SpoT model, persisters are cells with a higher level of ppGpp. The same group recently demonstrated that a ppGpp-dependent pathway is also involved under biofilm conditions Strikingly, they identified specificities regarding the importance of each involved protein or enzyme Finally, these results could lead to the design of antibacterial agents targeting the stringent response, such as RelA inhibitors, in order to increase persister cell mortality In , a connection was established between the SOS system and tolerance.
This event, which requires the SOS-promoting recA and lexA genes as well as dpiA , transiently halts bacterial cell division, enabling survival upon otherwise lethal antibiotic exposure A more recent study demonstrated that, in E. TisB can be inserted into the inner membrane and disrupt the proton motive force, which leads to a drop in the intracellular level of ATP. Subsequent shutdown of cellular processes is thought to be responsible for the observed higher level of persisters , Recently, it was shown that starvation and the SOS response can induce high biofilm-specific tolerance toward ofloxacin In that study, a screen for E.
It was demonstrated that both functional RecA and cleavable LexA were essential for the starvation-induced biofilm-specific ofloxacin tolerance phenotype and that the SOS response was induced upon biofilm aging concomitantly with ofloxacin tolerance Fig. Interestingly, a previous study from the same group showed that recA and other SOS response genes were significantly induced in mature biofilms compared to exponentially grown planktonic cells The latter results strengthen the notion that induction of ofloxacin tolerance in starving biofilms is likely to involve mechanisms different from those currently described for planktonic cells , It is noteworthy that the SOS system is also induced by conjugative DNA transfer, an event that is enhanced in biofilms Different defense mechanisms can be activated depending on the type of ROS.
As discussed above, antibiotic-induced oxidative stress might play an important role in bacterial cell death. Therefore, it was deemed plausible that a way for persisters to survive in the presence of bactericidal antibiotics was to protect themselves from oxidative stress. For instance, flow cytometer analysis demonstrated that in a population of antibiotic-treated E. Alongside the previously described stringent response-mediated defense against oxidative stress damages, another group reported that antioxidant strategies could lead to tolerance of bactericidal antibiotics.
Indeed, H 2 S has been demonstrated to increase the antioxidant capacity of Gram-positive and Gram-negative bacteria through suppression of the Fenton reaction and stimulation of SOD and catalase production Another group proposed a different scenario involving oxidative stress. They revealed that paraquat-induced oxidative stress led to an increase in the level of persisters surviving fluoroquinolone antibiotics, but not ampicillin or kanamycin Thus, exposure to lower concentrations of fluoroquinolones may lead to persister formation.
Lastly, it was shown that oxidative stress was induced in biofilms independently of the presence of antibiotics Therefore, it can be envisaged that in biofilms, due to an increased level of oxidative stress, the SOS response is induced and increases the level of tolerance, as demonstrated in the case of ofloxacin Aside from the above-described genetic mechanisms, different genes or regulators are involved in the switch to the persister state or, less precisely, in an increase in bacterial tolerance.
In most of the following cases, the precise links between these genes and tolerance are not known. Inactivation of the phoU gene leads to decreased tolerance toward a wide range of antibiotics and various stresses, such as acidic pH, starvation, and heat. In case of starvation, phoU is expressed and affects genes involved in energy production and membrane transport. The precise effectors through which PhoU suppresses cellular metabolic activity are not known.
Another group used survival of ampicillin treatment as a screening method for an E. Although deletion of glpD did not affect tolerance in exponential-phase cultures, it eliminated the majority of persisters in stationary phase. Two additional multidrug tolerance loci, glpABC and plsB , were identified through study of the pathway involving sn -glycerolphosphate metabolism.
The importance of quorum sensing QS signals in tolerance was demonstrated in P.
Introduction
The difference in tolerance might be related partly to a different biofilm structure, as QS plays an important part in biofilm architecture. However, similar observations were made in P. Pyocyanin, secreted by P. Another structurally related compound paraquat had a similar effect, whereas phenazinecarboxylic acid PCA did not, despite strong structural similarity.
AmgRS is a two-component regulator, and its mutation was identified through screening of tobramycin-susceptible mutants. Indeed, amgRS mutations reduce planktonic and biofilm tolerance toward aminoglycosides Transcription profiles suggest that AmgRS controls an adaptive response to membrane stress, possibly caused by aminoglycoside-induced insertion of misfolded proteins The possible effectors of AmgRS-induced tolerance may be membrane proteases HtpX and NlpD and a protease-associated factor YccA , which would help to eliminate misfolded proteins.
One mechanism that can be hypothesized for the persister switch is that of stochastic gene expression through fluctuations in transcription and translation rates despite stable environmental conditions These variations result from two types of noise: Indeed, even when all members of a planktonic culture are exposed to the same growth conditions, only a small fraction of them are persisters, suggesting the involvement of stochasticity In this case, we speculate that at the population level, there exists a mean level of key persister regulatory protein expression associated with intracellular fluctuations due to the noises.
For a small subset of bacteria, the level reaches a threshold, leading to the phenotypic switch Fig. Then, when the population meets environmental triggers inducing stringent or SOS responses, the basal level of expression increases, leading to an increase in the percentage of cells reaching the threshold 94 , This hypothesis has been supported by experiments performed with TA modules that also demonstrate that the amount by which the threshold is exceeded determines the duration of dormancy As demonstrated above, many pathways, molecular mechanisms, and environmental factors are involved in the phenotypic switch that leads a bacterium to become a persister.
Furthermore, some of these pathways are interconnected. Therefore, it is very likely that depending on the conditions prevailing during the switch, different types of persisters may appear, possibly simultaneously, in the same culture , The type of antibiotic used to eradicate nonpersisters is a striking example of this and can influence gene expression, SOS induction, and the oxidative stress defense.
Even with homogeneous stresses similarly affecting the whole population, it was demonstrated that both a growth-arrest-mediated pathway and ppGpp-dependent pathways can be activated, leading to different types of persisters Ten years ago, the study of persisters by use of a microfluid device led to the hypothesis that two main types of persisters were produced: It is now clear that this view caught only a glimpse of the complexity and diversity of persisters. Although most of the above-mentioned mechanisms were discovered under plankonic conditions, it is very likely that they are also involved in the generation of persisters in biofilms.
For instance, due to nutrient limitations, the stringent response has already been shown to play a central role in P. The SOS response is induced in biofilms and plays a role in biofilm recalcitrance toward antibiotics On the other hand, due to the existence of biofilm-specific phenotypes and functions, caution should be taken in extrapolating persister data obtained under planktonic conditions to the biofilm lifestyle. Indeed, biofilm-specific mechanisms have been described and underline the complexity in the study of persisters Patients suffering from biofilm-related infections are also exposed to nosocomial microorganisms present in their health care environment and selected by repeated antibiotic treatments.
In this case, biofilm formation and gene resistance issues can be additive as well as synergistic. Biological processes involved in horizontal gene transfer, such as conjugation, transformation, and transduction, have been demonstrated to be increased in vitro in biofilms for a comprehensive review of this issue, see reference Furthermore, while the presence of conjugative plasmids promotes biofilm formation, the biofilm lifestyle also increases plasmid stability and the range of mobile genetic elements Hence, the presence of a biofilm is expected to facilitate the transfer of resistance genes, as demonstrated in an in vitro study, with an increased rate of transfer of a plasmid encoding CTX-M an ESBL in a K.
Transfer of a conjugative transposon Tn carrying antibiotic resistance might also be responsible for acquisition of resistance mechanisms in biofilm bacteria Transferability of genetic mobile elements between bacteria belonging to a multispecies biofilm has been described for a medical device implanted in a patient Interestingly, many transmissible DNA elements encode biofilm-promoting factors, including adhesins, such as conjugative pili, fimbriae, and autotransporter adhesins, and persistence factors, such as toxin-antitoxin modules.
For instance, the F-plasmid-based CcdAB TA system increases the persister level and thus constitutes a transmissible persistence factor Due to biofilm architecture and drug diffusion issues, it is likely that some biofilm areas may be submitted at least transiently to subinhibitory concentrations of antibiotics. Exposure to subinhibitory concentrations of antibiotics is known to increase the likelihood of selecting resistant mutants for a comprehensive review, see reference Although it is generally assumed that selection of resistant bacteria occurs at antibiotic concentrations between the MIC of the susceptible wild-type population and that of the resistant bacteria, recent studies suggested that such selection could also occur at lower antibiotic concentrations Furthermore, bacteria may produce hydroxyl radicals when exposed to sublethal concentrations of antibiotics These hydroxyl radicals can induce the occurrence of mutations and help the organism to acquire resistance mechanisms.
Similar findings have been made in P. Exposure to tobramycin at subinhibitory concentrations can increase the c-di-GMP level and biofilm formation, as demonstrated in E. Similar findings were made upon exposure of Corynebacterium diphtheriae to subinhibitory concentrations of erythromycin and, to a lesser extent, penicillin , but also for P. Recent studies also reported that antibiotics at subinhibitory concentrations can promote the transfer of mobile genetic elements, even though this has been demonstrated primarily under planktonic conditions.
For instance, the fluoroquinolone-mediated SOS response may trigger expression, excision, and transfer of prophage genes SOS induction may promote mobilization of various mobile elements, such as integrating conjugative elements It has been shown that conjugation induces the SOS response and promotes antibiotic resistance through integron integration and activation in vitro , Finally, as previously discussed, ciprofloxacin has been shown to increase the frequency of persisters through induction of SOS and, ultimately, production of the TisB toxin , In general, preexposure to subinhibitory concentrations of antibiotics 0.
Although most of the data discussed here were generated with planktonic bacteria, it can be envisaged that this phenomenon is relevant in the case of reduced diffusion of antibiotics through the biofilm matrix. Because biofilm persisters are more likely to survive antibiotic treatment, they are exposed to repeated rounds of different classes of antibiotics, inducing all the above-mentioned consequences and thereby amplifying the phenomenon Although the interplay between biofilm recalcitrance, gene transfer, and spread of resistance could be of key importance in nosocomial settings, it remains to be demonstrated in clinical settings, or even in a relevant in vivo model of biofilm-related infections.
Various examples of genetic diversity occurring in biofilms have been described as influencing biofilm tolerance toward antimicrobial agents.
Genetic variants arise when breaks are repaired by a mutagenic mechanism involving recombinatorial DNA repair genes. Several genes, such as katA and sodB , also shown to be involved in protection against oxidative DNA damage, were downregulated under biofilm conditions A similar mechanism has been described for the mucoid conversion of P.
Hypermutators have been identified in clinical samples, and some of them are associated with specific mutations, such as mutS , belonging to the DNA mismatch repair MMR system Aside from mutL and uvrD , which also belong to the MMR system, other genes were found to be mutated in hypermutators, such as mutT , mutY , and mutM , belonging to the DNA oxidative lesion repair system , — Similar findings have been made in staphylococci, with mutability in biofilms that is fold S.
These mutations can lead to tolerance or resistance mechanisms. SCV constitute a subset of the bacterial population that has been identified in a wide range of bacteria, including S. They are associated with many diseases, including biofilm-related infections, such as osteomyelitis, chronic pulmonary infections in CF patients, and device-related infections.
It has been demonstrated that their slow growth originates mainly from mutations associated with two types of defect: These SCV are frequently auxotrophic and are less susceptible to various antibiotics, depending on the metabolic alterations they exhibit for comprehensive reviews of these issues, see references and As SCV may be present in biofilms, they may be involved in the global recalcitrance of the bacterial community.
When RSCV are grown on antibiotic-free agar, wild-type revertants with a large colony size and a smooth appearance arise on the edges of the variant colonies. The regulatory protein PvrR of the two-component system PvrSR has been found to control conversion between antibiotic-resistant and antibiotic-susceptible forms. Even prior to identification of the link between biofilms and human diseases, different therapeutic strategies were developed to prevent the occurrence of microbial colonization and to eradicate device-related infections, once established.
However, most developments in the field of antimicrobial agents were based on planktonic studies, without taking into account the specificities of the bacterial biofilm lifestyle. Although hygiene is not a specific antibiofilm strategy, it prevents microbial contamination and thus adherence and subsequent biofilm formation. For almost all types of device-related infections, guidelines have been proposed to standardize procedures for device implantation and handling.
For instance, the insertion of any central venous catheter CVC must be performed by trained personnel with maximum sterile barrier precautions, defined by the use of sterile gloves, cap, mask, sterile gown, and a sterile full-body drape , The choice of skin disinfection solution and methods is also of key importance, and many reports suggest that alcohol-based antiseptics, such as alcohol-based chlorhexidine and alcohol-based povidone-iodine, are the most efficient solutions Improvement of hygiene measures should always be attempted through definition and implementation of local clinical bundles for device insertion and handling, and in the case of CVC, dedicated infusion therapy teams have been developed for the education of patients and health care workers , , Once a device is removed, the risk of bacterial contamination drops to zero.
Therefore, at any time, physicians must discuss the benefits of maintaining an indwelling foreign body. For instance, a meta-analysis reported that use of an automatic reminder system for the removal of useless urinary catheters significantly decreased the incidence of catheter-associated urinary tract infections CAUTI Of course, this approach is more difficult in the case of mandatory devices, such as pacemakers. Depending on the type of device, systemic antibiotic prophylaxis can be proposed in order to reduce the risk of microbial contamination.
In that case, antibiotics are injected a few minutes before skin incision and are dedicated to eradicating any microorganisms that are not removed by skin disinfection. This approach is recommended in the case of surgically implanted devices, such as orthopedic and cardiac devices , The principle of antibiotic coating of implanted devices is to deliver a locally high concentration of antimicrobials at the site of potential colonization Depending on the type of device and the length of implantation, these antibiotic-coated materials can efficiently reduce the rate of colonization.
The example of CVC can be taken to illustrate the benefits and limits of the antibiotic coating strategy. Two types of coating have been developed: Comparative studies concluded that the former is more efficient than the latter , — However, the benefits of antibiotic coating for long-term intravenous catheters LTIVC have not yet been demonstrated.
Indeed, as these devices dwell for longer periods, the surfaces of LTIVC will be covered by a conditioning film composed of host cells or proteins that might limit the effect of any active surface. Furthermore, as soon as the antibiotic contained in the device is exhausted, antibiotic delivery stops.
Antibiotic-coated surfaces have also been studied in animal and clinical studies of urinary catheters, endotracheal tubes, orthopedic devices, and vascular grafts, with contrasting clinical benefits , — Thus, development of a coated surface that prevents bacterial colonization for a long time remains a challenge. When clinicians are confronted with therapeutic difficulties or local and systemic complications, removal of the indwelling device may be required in the case of biofilm-related infection , , , For short-term peripheral catheters, removal and replacement are easy, painless, and inexpensive.
In contrast, removal of long-term catheters, pacemakers, or orthopedic prostheses is associated with complications for the patient, as well as with high costs.
INTRODUCTION
In the case of tissue-related infections, surgical removal of biofilm may be indicated for antibiotic failure. This is particularly the case for infective endocarditis IE and osteomyelitis, during which failure to cure the infection is an indication for surgery As physicians and clinical microbiologists became more aware of the importance of biofilms in infectious diseases, they attempted to define the antibiotics that were most active against biofilms and how these antibiotics should be prescribed so as to increase the likelihood of infection eradication.
One famous example of this challenging process is that of the rifampin-containing regimen, demonstrated to significantly improve the outcome of foreign-body-related S.
Furthermore, fosfomycin and daptomycin are currently being investigated and might be promising candidates in the fight against foreign-body-related infections , — In the case of prosthetic joint-related infection PJI , in vivo models led to the demonstration that fluoroquinolones exhibited more penetration into the site of infection Furthermore, in vivo models of foreign-body-related infections demonstrated that fluoroquinolones were the most efficient molecules when associated with rifampin Based on these findings, fluoroquinolones have now become one of the mainstay treatments of PJI A more recent example of an antibiotic associated with a potent antibiofilm effect is that of daptomycin.
This bactericidal cyclic lipopeptide has an in vitro spectrum against Gram-positive pathogens through calcium-dependent disruption of membrane function, leading to potassium ion leakage and inhibition of DNA, RNA, and protein synthesis , In vitro studies suggested that daptomycin may quickly penetrate S. However, daptomycin alone was not able to cure the infection caused by methicillin-resistant S. Nevertheless, these 2 antibiotic combinations were more efficient than the previously recommended vancomycin-rifampin and linezolid-rifampin combinations Using a similar methodology, another group demonstrated that daptomycin or rifampin as a single agent against MRSA was more effective than vancomycin or linezolid Daptomycin has also been proposed for the treatment of catheter-related infections, and an in vivo study demonstrated that vancomycin and daptomycin were equally efficient at eradicating methicillin-resistant S.
Comparative clinical studies are now expected to determine, for instance, whether daptomycin is more efficient and more rapid than vancomycin.
Biofilms, Infection, and Antimicrobial Therapy | Taylor & Francis Group
In the case of P. In that case, the early association of oral ciprofloxacin with inhaled colicin is associated with a reduced risk of chronic colonization , In addition to the choice of specific antibiotics, high dosages and prolonged treatment courses are required for biofilm-associated infections, as emphasized by cases of IE and osteomyelitis , This solution should dwell for an extended time at least 12 h in order to eradicate any incoming bacteria.
The chosen volume must allow coverage of the entire internal surface and therefore depends on the type of device, but it is usually small between 2 and 5 ml. Indeed, microbial contamination of LTIVC occurs on the inner side of the device, defining intraluminal colonization. Thus, the highly concentrated antibiotic solution will be in close contact with the biofilm. On the other hand, in case of short-term CVC, contamination occurs mainly on the external surface of the device, defining extraluminal contamination.
In that case, ALT cannot access the biofilm and is therefore useless. Other groups also assessed the combination of an antibiotic minocycline and a chelator, such as EDTA. Nevertheless, systematic use of ALT could lead to increased antibiotic resistance and should therefore be considered only for high-risk patients who have already experienced LTIVC-related infections , , On the other hand, limited data are available concerning nonantibiotic lock solutions, such as ethanol and taurolidine, but they might also be used among high-risk patients , Indeed, if the clinical situation allows, catheter salvage is indicated in cases of reduced venous access or the potential presence of coagulation disorders Such conservative treatment could avoid risks and reduce costs associated with a new surgical procedure.
In other cases, conservative treatment using a combination of systemic antimicrobials and ALT can be considered 90 , Despite several limitations, there is a growing body of evidence favoring the use of ALT. For instance, a randomized, placebo-controlled study showed that ALT plus systemic antimicrobial therapy is more effective than systemic antimicrobial therapy alone for treating LTIVC-related BSI, although the result did not reach statistical significance due to the small sample size Aside from commonly used antimicrobials in ALT, ethanol and daptomycin have recently been used for conservative treatment see the previous section for daptomycin data.
However, clinical data are still needed in order to recommend ethanol as a first-line compound for ALT , — Currently used strategies have clearly improved the management of patients with indwelling devices in terms of both prevention and treatment of biofilm-related infections. However, many challenges remain before we can decrease the risk of microbial contamination on a surface or increase the likelihood of biofilm eradication.
It is very likely that specific targeting of mechanisms known to play a role in biofilm recalcitrance will be a relevant strategy. Within the limits of the different preventive approaches and the fact that most of them rely on the use of antibiotics, many efforts have been made to identify preventive strategies based on fundamental knowledge of mechanisms involved in bacterial adherence and biofilm formation Fig. Antibiofilm strategies arising from fundamental research. Approaches to preventing formation of biofilms are depicted in blue; approaches to eradicating an established biofilm are shown in red.
Persister cells are shown in red. Given the fact that without initial adhesion a biofilm cannot develop, the objective of inhibiting microbial adhesion is to impede the initial steps in biofilm formation. Inhibition of microbial adhesion to surfaces has been discussed extensively in several reviews , — Here we simply describe the main approaches and develop a relevant example of each. Since initial adhesion implies bacterial and surface factors, the physicochemical characteristics of the surface are of key importance in prevention of device-related infections. The physical nature of the material is important, as illustrated by human models of dental implant-associated biofilm This experimental approach was used to demonstrate that bacterial adhesion to implant surfaces is significantly lower with a zirconium oxide surface than with pure titanium Ti The biomaterial manufacturing process can also modify roughness and physicochemical properties and thus affect bacterial adhesion.
Indeed, electropolished stainless steel reduces bacterial adhesion compared to that with sandblasted steel Furthermore, the choice of the polymeric material, even without any modification, is of key importance. Using a high-throughput microarray assay, bacterial adhesion was assessed on hundreds of polymeric materials and led to identification of materials comprising ester and cyclic hydrocarbon moieties Coating of silicone with these materials significantly decreased S.
The physical architecture of the surface can also help to prevent microbial adhesion. For instance, the sharklet micropattern is a surface modification that mimics the microtopography of shark skin and has been shown to significantly reduce Gram-negative bacterial adhesion in vitro , Another major strategy for reducing bacterial adhesion is to modify the surface so it is protected by grafting antiadhesive molecules. One limitation to coated devices lies in the progressive coverage by a conditioning film made of proteins or cells from the patient. Thus, different attempts have been made to reduce not only microbial adhesion but also the deposition of host components or the occurrence of thrombosis.
To do so, a peptide-based coating technology was proposed to modify the surface of Ti metal through noncovalent binding Another group used lysozyme immobilized on polyethylene glycol monomethacrylate PEGMA to coat stainless steel surfaces and demonstrated that bacterial adhesion and albumin adsorption were reduced Another surface modification using zwitterionic a molecule with both positive and negative charges nonleaching polymeric sulfobetaine polySB was associated with significant reductions in adherence and activation of platelets and white blood cells This scaffold retains water on the surface of the catheter surface and reduces not only protein, host cell, and microbial adhesion but also thrombus formation in vitro and in vivo Although these approaches have produced encouraging results, they still need to be evaluated in long-term settings.
Other surface modifications have been designed to kill bacteria once they stick to the surface, without using antibiotics. Two examples can be presented. First, poly 4-vinyl- N -alkylpyridinium bromide covalently attached to glass slides and immobilizing polycationic chains that have antibacterial properties is able to kill airborne bacteria on contact Second, single-walled carbon nanotube SWNT coatings were reported to have antimicrobial activities through cell membrane perturbation after an initial SWNT-bacterium interaction that ultimately leads to electronic structure-dependent bacterial oxidation and death Biosurfactants are surface-active molecules produced by many bacteria to inhibit adhesion of competitors.
Such molecules have therefore been studied as a possible surface modification in order to prevent bacterial adhesion. For instance, group 2 capsule and Ecp, two hydrophilic high-molecular-weight polysaccharides produced by different E. Other molecules have been tested, including surfactin, rhamnolipids, and other molecules produced by lactobacilli and Streptococcus thermophilus , although these have not been identified clearly see the reviews in references and Because of limitations related to antibiotic coatings, such as their effect being restricted to nonresistant bacteria, different groups have tried to identify nonantibiotic coatings for preventing microbial adhesion.
Use of antibody-releasing surfaces, such as a biomedical-grade polyurethane hydrogel coating containing solid dispersed bioactive antibodies, was proposed The presence of antibodies reduced bacterial adhesion and enhanced bacterial killing during an in vitro opsonophagocytic assay using freshly isolated blood neutrophils IgG opsonization was shown to inhibit bacterial adhesion by blocking cell surface attachment factors and altering the surface hydrophobicity of the bacterial cell Since nitric oxide NO has antibacterial properties, NO-releasing surfaces have been proposed.
An in vivo model using a medical-grade silicone elastomer with an NO-storing film implanted in rats led to a reduction in bacterial colonization after S. Other vascular catheter coatings have been studied, such as the association of triclosan an antiseptic and dispersin B an antibiofilm enzyme see below to prevent S. Triclosan-loaded urinary catheters have also been studied successfully in vivo for the prevention of Proteus mirabilis CAUTI Another antiseptic-coated catheter containing gendine demonstrated significant reductions in E. Different molecules have been designed to specifically inhibit the production of bacterial adhesins involved in biofilm formation.
As an example, ring-fused 2-pyridones inhibit curli biogenesis in uropathogenic E. Some of them also have a pilicide effect, i. One molecule, FN, has been demonstrated to block biogenesis of both curli and type 1 pilus, to inhibit biofilm formation, and to attenuate virulence in a mouse model of urinary tract infection Another approach is to specifically target the FimH type 1 pilus lectin of UPEC, which mediates bacterial colonization, invasion, and formation of recalcitrant intracellular bacterial communities in the bladder epithelium Low-molecular-weight mannose-derived compounds called mannosides were designed and adapted for oral administration.
Indeed, the mannose binding pocket of FimH is composed of amino acids that are invariant in all strains of E. When tested in a mouse model, the mannosides were able to prevent UTI when given prophylactically or to treat an established chronic urinary tract infection Furthermore, synergistic action was noted between mannosides and trimethoprim-sulfamethoxazole, suggesting the utility of adjuvant approaches in this setting Prophylactic administration of a mannoside molecule, compound ZFH, was recently demonstrated to significantly reduce bacterial colonization of the bladder and to prevent acute UTI caused by an epidemic multidrug-resistant UPEC ST clone.
Treatment of chronically infected mice with the same FimH inhibitor lowered their bladder bacterial burdens over 1,fold Aside from direct administration of an adhesin inhibitor, other authors proposed covering a surface with an adhesin inhibitor. For instance, coverage of a surface with multivalent galabiose derivatives significantly inhibits adhesion of E. The main limitation of this approach is the multiplicity of structures involved in bacterial adhesion.
Biofilms, Infection, and Antimicrobial Therapy
However, one way to circumvent this issue is to use multivalent inhibitors linked to a scaffold of glycopolymers, glyconanoparticles that may permit inhibition of several adhesins at the same time , — Lactoferrin is a component of innate immunity found in numerous body fluids tears, milk, and respiratory secretions and is an iron chelator that has been demonstrated to inhibit irreversible adhesion of P.
Through iron chelation, lactoferrin stimulates twitching motility, during which bacteria wander across the surface instead of forming microcolonies and biofilms. Indeed, iron metabolism and transport are required for normal biofilm development The effect of lactoferrin can be increased by the adjunction of xylitol, a rare sugar that inhibits the ability of the bacteria to produce siderophores under conditions of iron restriction Such an association could be proposed in case of chronic wounds colonized by P.
Assessment of the antibiofilm efficacy of other known iron chelators and development of new iron chelators targeting biofilms might be future antibiofilm strategies to consider. The inhibition of c-di-GMP biosynthesis by diguanylate cyclase DGC is also promising, in light of its importance in the shift from the planktonic to the biofilm lifestyle. Indeed, blocking c-di-GMP biosynthesis may keep bacteria in the planktonic state. Screening for DGC inhibitors identified sulfathiazole Sulfathiazole inhibits formation of biofilms in vitro and indirectly inhibits DGC through inhibition of tetrahydrofolate biosynthesis, which affects the pool of thymidine, and DNA synthesis, rather than via enzymatic inhibition , More direct inhibition of DGC was identified in V.
Several molecules inhibiting DGC and biofilm formation of these three pathogens were identified; however, the tolerance and toxicity of most of these compounds remain to be assessed. Physical approaches have been developed to prevent biofilm formation on catheters, including low-energy surface acoustic waves and iontophoresis as preventive measures , In the latter case, urethral catheters are modified in order to deliver a current to electrodes located on the catheter tip, leading to production of ions of soluble salts and allowing formation of a local biocide.
After 3 weeks, this approach significantly reduced the bacterial burden in urine. Surface acoustic waves have also been proposed for the eradication of biofilms, in conjunction with antibiotics The objective of the jamming approach is to inhibit biofilm formation by altering the progression from initial attachment to microcolonies and development of a mature biofilm. As quorum sensing QS is a key component of biofilm communication, many authors have speculated that interfering with QS signals might alter biofilm maturation, thereby leading to easier eradication. However, the main limitation of QS inhibition is the spectrum of action, which depends on the type of QS system used by the microorganism responsible for the infection.
Similar in vitro and in vivo data have been published for S. Azithromycin has poor antimicrobial activity against P. It was shown to inhibit P. Clinical studies in CF patients colonized by P. Nevertheless, recent data suggest that the chronic use of azithromycin might be associated with side effects, such as ototoxicity and an increased level of bacterial resistance , Possible cardiovascular toxicity has been described, with conflicting results As acyl-homoserine lactones AHLs play a key role in the development of P.
An N -acyl-homoserine lactone hydrolase BpiB05 was identified through screening of a soil metagenome, and it inhibits P. Along with AHLs, synthetic furanones derived from an algal metabolite now constitute potential prevention candidates, as they inhibit Gram-negative bacterial QS through their fixation to LasR and inhibition of the action of AHLs , In vitro and in vivo studies reported reduced biofilm formation, virulence factor expression, and antibiotic tolerance of P.
However, furanones have a narrow spectrum of activity, as they are efficient only against bacteria that share this QS signaling pathway , Furthermore, the use of halogenated furanones remains hampered by their carcinogenic effects as well as poor stability in aqueous solutions. Through screening of chemical libraries, different QS inhibitors have been identified, such as garlic extract and 1-isothiocyanato methylsulfinyl propane, also known as iberin, from horseradish , The compound isolated from garlic, ajoene 4,5,9-trithiadodeca-1,6,trieneoxide , was shown to increase P.
In vivo , mice treated with garlic extract for 7 days, with the initial 2 days being given before P.
- Simply Beautiful, Rooted in Him;
- Playing with the Past: Digital Games and the Simulation of History.
- 1st Edition?
Another example of a QS inhibitor identified in vegetal matter is green tea epigallocatechin gallate, which was shown to reduce QS, biofilm development, and virulence factor production of P. Lastly, different authors have proposed grafting enzymes able to digest QS signals, called quorum-quenching molecules, on the surface in order to inhibit bacterial adhesion , The goal of vaccination is to induce the production of antibodies against bacterial biofilm antigens, such as structures involved in adhesion or biofilm maturation. This strategy requires predefining groups of patients about to be exposed to the risk of biofilm-associated infection and treating them before exposure.
A relevant example is the scheduled implantation of devices such as heart prosthetic valves, pacemakers, and prosthetic joints. This strategy may also be relevant for patients exposed to chronic tissue-associated infections, such as CF patients or patients suffering from recurrent UTI. Ideally, biofilm-specific antigens should be used to increase the effect of vaccination.
- Acknowledgments;
- Biofilms, Infection, and Antimicrobial Therapy - CRC Press Book.
- Herbs Tips for Living.
Choosing the right antigen remains an arduous task due to the obvious redundancy of bacterial appendages involved in adhesion and biofilm formation. Therefore, current strategies are aimed at using an antigenic cocktail , For CVC-related infections, a rat model enabled assessment of immunization of rats prior to catheter insertion, leading to a protective effect in bacterial colonization of the device by S.
In that study, two different antigens were used: Aside from vaccination aimed at preventing bacterial adhesion, it has also been suggested that vaccination will increase the likelihood of biofilm eradication Antigens were chosen glucosaminidase, an ABC transporter lipoprotein, a conserved hypothetical protein, and a conserved lipoprotein because they are upregulated in biofilms both in vitro and in vivo.
In a rabbit osteomyelitis model, the association of antibiotics and vaccination significantly increased the rate of therapeutic success In that model, vaccination was initiated 30 days prior to the onset of infection, thus reducing the impact of the findings. The use of nonpathogenic bacteria to prevent colonization relies on nonpathogenic bacteria that are able to efficiently colonize a surface and thus compete with other bacterial pathogens and prevent their adhesion The best-documented case is the E.
This strain lacks most virulence factors and UTI-associated adhesins and fails to induce bladder inflammation It was observed that antibiotic treatment of patients with ABU led to a paradoxical increase in the risk of UTI by other bacteria, thus leading to the hypothesis that E.
Since then, different clinical studies have demonstrated that bladder inoculation with E. For instance, in a clinical pilot study, patients with incomplete bladder emptying and recurrent UTI were randomized to receive blinded bladder inoculations with E. Inoculated patients experienced significantly fewer UTI during the 12 months following inoculation. Hence, several promising strategies have been developed to prevent microbial adhesion and biofilm formation. Only a few of them have undergone in vivo efficacy tests, and for most of them, the precise mechanisms of action remain unknown Table 2.
Most currently used strategies for biofilm eradication were developed even before the identification of the importance of biofilms in human medicine. Therefore, major improvements have already been made in these fields. However, several therapeutic failures are still being observed, even when patients are managed at reference centers.
Furthermore, most currently used strategies rely on antibiotics, thereby increasing the selective pressure and the risk of antibiotic resistance. Finally, prolonged treatment is frequently required, leading to considerable medical cost and toxicity. Inducing dispersal is a tempting strategy; indeed, biofilm bacteria lose some of their antibiotic tolerance when they return to a planktonic state and are exposed to the host immune system However, the dispersal approach needs to be associated with the use of systemic antibiotics, as release of biofilm bacteria into the bloodstream can lead to severe sepsis , Several strategies have been proposed to induce biofilm dispersal.
Because ECM plays an important role in maintaining biofilm stability and structure, it has been speculated that use of an enzyme able to dissociate or digest ECM components would lead to dispersal of the biofilm. Two main targets have been identified: It is therefore effective against biofilms formed by this bacterial species , On the other hand, given the important role played by extracellular DNA in the structure of the biofilm matrix , , DNase I, an enzyme that degrades DNA, was efficiently used to dissolve biofilms from a broad range of bacteria, including P.
However, the enzyme-based approach is associated with two limitations: Since divalent cations play a key role in maintaining biofilm ECM stability and cohesiveness, another approach is to use chelators such as EDTA and citrate , For instance, calcium ions cross-link alginate, and the calcium concentration was shown to be critical for maintaining P. Iron has also been demonstrated to be an important cross-linker of the ECM However, little is known about the mode of action and precise effects of chelators on biofilms.
One study in reported that the addition of EGTA, a specific calcium chelator, led to immediate detachment of a mixed bacterial film from the walls of a recycle tube reactor Strikingly, addition of calcium, iron, or magnesium inhibited the phenotype. In that study, EGTA led to the same dispersal phenotype, but without inducing lysis. Furthermore, citrate and EDTA were also shown to exhibit direct bactericidal effects against planktonic bacteria , Gentamicin-EDTA is therefore a potential lock solution able to cure highly tolerant biofilms and eradicate persistent bacteria, thereby preventing recurrence of Gram-positive as well as Gram-negative bacterial biofilms on TIVAP While QS signaling can be targeted to interfere with biofilm formation, some QS signals can also be used to trigger dispersal of a biofilm.
