Additionally, the in vivo functioning of a regenerated kidney remains unclear. However, further advances in developmental biology and bioengineering may resolve these issues and allow whole kidney regeneration. Injection of PSCs into blastocysts, the initial embryonic stage after fertilization, synchronizes the development of two line cells, and the combined blastocyst generates a chimeric body. In the first report of this method, normal ESCs were injected into blastocysts of recombination-activating gene 2-deficient mice, which have no mature B or T lymphocytes, to generate somatic chimeras with ESC-derived mature B and T cells [ 28 ].
Bioengineering Strategies for the Treatment of Type I Diabetes.
A recent study to generate a functional organ using blastocyst complementation by Kobayashi et al. The mouse and rat PSC-derived pancreas produced insulin, and the transplantation of PSC-derived pancreas islets improved hyperglycemia in a diabetic rodent model [ 37 ]. This study indicated that PSC-derived cellular progeny could occupy and develop in a vacant developmental niche. Furthermore, these results also demonstrated that interspecific blastocyst complementation could be used to generate organs derived from donor PSCs in vivo using a xenogeneic environment [ 36 , 37 ].
This blastocyst complementation system has already been applied to whole kidney reconstruction [ 36 ]. Nondeficient murine iPSCs were injected into blastocysts from kidney-deficient mice lacking the SAL-like 1 protein essential for kidney development, and the neonatal mice had kidneys derived almost entirely from injected iPSCs [ 36 ].
However, the vascular and nervous systems were not constructed from cells of iPSC origin, and the kidney was therefore not completely complemented. Immunohistochemical analysis of the regenerated kidney indicated that the renal vascular system, including renal segmental, lobar, interlobar, arcuate, and interlobular arterioles, was a chimeric structure originating from both host cells and donor iPSCs [ 36 ]. Precise urinary analysis was not carried out and whether or not filtrated and reabsorbed urine was produced is unclear.
Moreover, injection of rat iPSCs into kidney-deficient mouse blastocysts failed to generate rat kidneys in mice. This result implies that the key molecules in mice involved in interactions between the mesenchyme and UB do not cross-react with those in rats. The generation of xenogeneic organs using interspecific blastocysts thus requires a host animal strain lacking all renal lineages [ 36 ].
The most important problem associated with blastocyst complementation is the ethical issue. It is impossible to exclude the possibility of generating interspecific chimeras containing brain derived from injected PSCs. Although it is difficult to establish a xenogeneic blastocyst complementation system that overcomes the xenogeneic barrier, this strategy appears to be one of the most promising methods for kidney regeneration. The clusters from dissociated S3-segment cells were induced by the hanging-drop method in 3D culture [ 44 ], while 2D culture conditions were unable to reconstruct kidney-like structures.
Surprisingly, the reconstructed kidney-like structures included all the kidney substructures, including glomeruli, proximal tubules, the loop of Henle, distal tubules, and the collecting ducts, but not the vasculature. They assumed that these cells were similar to metanephric mesenchymal cells, based on marker protein expression. However, the clusters can differentiate into collecting-duct-like cells or mesangial-like cells, which are not thought to be derived from MM [ 44 ].
In this regard, the question of whether adult kidney stem cells can differentiate into lineages other than UB or MM remains to be answered. The kidney-like structures were not vascularized and did not produce urine. However, adult kidney stem cells remain poorly understood. These reports raise the possibility that adult stem cells may represent a safer clinical source than PSCs.
Native kidney extracellular matrix ECM has been reported to provide a scaffold for cell seeding and a niche for stem cells to differentiate into whole organs [ 48 ]. The ECM plays a crucial role in kidney development and repair [ 48 — 52 ]. ECM molecules and their receptors influence organogenesis and repair by providing a scaffold for the spatial organization of cells, by secreting and storing growth factors and cytokines, and by regulating signal transduction [ 48 — 53 ].
ECM scaffolds from whole human-cadaveric and animal organs can be generated by detergent-based decellularization [ 1 , 54 ]. This strategy was used by Ott et al.
A whole-heart scaffold with intact 3D geometry and vasculature was prepared by coronary perfusion with detergents into the cadaveric heart. The rat heart was then seeded with neonatal cardiac cells or rat aortic endothelial cells, which subsequently induced the formation of contractile myocardium that performed stroke work [ 55 ]. Decellularized cadaveric scaffolds have also been used in several other organ systems, including the liver [ 56 ], respiratory tract [ 57 ], nerves [ 58 ], tendons [ 59 ], valves [ 60 ], bladder [ 61 ], and mammary gland [ 62 ].
Furthermore, some studies have used decellularization-recellularization technology for kidney regeneration. Many animals have been used for decellularization studies, including rats [ 63 ], rhesus monkeys [ 64 ], and pigs [ 65 ].
Current Bioengineering Methods for Whole Kidney Regeneration
However, regenerated kidneys produced by this method did not have sufficient renal function to produce urine and Epo. Notably, they generated 3D acellular renal scaffolds by perfusion decellularization of cadaveric rat, pig, and human kidneys. Endothelial and epithelial cells were repopulated by perfusion, leading to the formation of viable tissues for renal construction. However, the mechanism whereby the infused cells differentiate and are orchestrated into nephrons with vasculature to produce urine remains unclear.
Decellularized cadaveric scaffolds are associated with the problem of massive thrombi, despite strong anticoagulation prophylaxis. Although this strategy still has many obstacles, it demonstrates the impact of regenerative medicine on organ transplantation and its potential as a solution for the shortage of donor organs. The developing field of tissue engineering is an extension of cell therapy, in which biological and engineering science techniques are combined to create structures and devices to replace lost tissue or organ functions [ 67 , 68 ].
The development of bioartificial kidneys BAKs represents the intersection between regenerative medicine and renal replacement therapy [ 52 ]. A renal tubule assist device RAD containing living renal proximal tubule cells has been successfully engineered, and it demonstrated differentiated absorptive, metabolic, and endocrine functions similar to normal kidneys in animal experiments in vitro and ex vivo [ 69 ]. Briefly, renal proximal tubule segments were harvested from kidneys, and renal tubule progenitor cells were selected and expanded [ 70 ].
The tubule progenitor cells were grown in culture dishes with culture medium containing specific additives [ 68 ]. A RAD with high-flux hemofiltration cartridges containing polysulfone hollow fibers coated with pronectin-L was used as a scaffold device [ 68 ]. Renal proximal tubule cells were then seeded into the hollow fibers and the seeded cartridge was connected to a bioreactor perfusion system, in which the extracapillary space was filled with culture medium and the intracapillary space was perfused with medium.
The cell cartridges were used at least 14 days after seeding. The RAD units included confluent monolayers of renal proximal tubule cells with characteristics including microvilli, tight junctional complexes, and endocytic vesicles demonstrated by transmission electron microscopy [ 68 ]. The tissue-engineered bioartificial RAD performed differential reabsorption and secretory transport because of the specific active transporters present in the proximal tubule cells in vivo.
However, these transport functions were less efficient than those in native proximal tubules [ 68 ]. The same group reported that the RAD was able to maintain viability in a uremic environment in uremic dogs with acute renal failure when placed in series with a conventional hemofilter and an extracorporeal blood circuit [ 71 ]. Furthermore, they also performed clinical trials with BAKs [ 72 — 74 ]. The combination of regenerative medicine and bioengineering thus offers promise for the regeneration of whole kidneys. We attempted to regenerate a functional, transplantable whole kidney able to produce urine and renal hormones, such as Epo, using a xenoembryo and human mesenchymal stem cells.
The opportunity of stem cell bioengineering.
The embryonic metanephros, which is the mammalian renal anlagen, is thought to represent a potential source for the regeneration of functional whole kidneys [ 75 — 83 ]. An embryonic metanephros transplanted into a host animal rat was able to obtain its blood supply from the host [ 75 ].
Indeed, the survival of anephric rats was prolonged on the basis of renal function provided by a single transplanted rat renal anlagen, the ureter of which was anastomosed to a host ureter [ 83 ]. Furthermore, the transplanted metanephros produced renal hormones including Epo and renin, which elevated the blood pressure of the host animal [ 77 , 78 ]. Metanephroi from porcine embryos implanted either in the omentum of mice in which costimulation was blocked [ 79 ] or under the kidney capsules of immunodeficient mice [ 81 ] developed fully functional nephrons. The levels of urea nitrogen and creatinine were higher in cyst fluid produced by the transplanted metanephroi than in sera from the transplanted host animals [ 81 ], suggesting urine production.
Metanephros transplantation was also shown to reduce vascular calcification in rats with chronic renal failure [ 79 ] and to maintain blood pressure in anephric rats with induced acute hypotension [ 78 ], implying that the transplanted metanephroi carried out multiple renal functions, other than urine production.
These results suggest that metanephros transplantation might be used to overcome the shortage of donor kidneys available for transplantation. We recently demonstrated that xenotransplanted metanephros could supply endogenous MSCs with a niche for differentiation into Epo-producing tissues [ 84 ]. Polymerase chain reaction using species-specific primers and sequence analysis revealed that xenotransplanted metanephroi, either from rat to mouse or from pig to cat, expressed Epo of host animal origin. This indicated that the Epo-producing cells originated in the host animal and developed to produce Epo in the transplanted metanephros.
We further showed that the Epo-producing cells did not originate from integrating vessels, but rather from circulating host MSCs mobilized from the bone marrow. Of note, conventional metanephros transplantation requires continuous and strong immunosuppression to avoid humoral rejection associated with the xenogeneic barrier, which can induce adverse effects including carcinogenicity and severe rejection. For safety purposes, the xenotransplant should thus be discarded when it is no longer required, by introducing a cell-fate-regulating system including a suicide gene that can be expressed on demand.
To avoid the xenogeneic barrier, we used metanephroi isolated from transgenic ER-E2F1 suicide-inducible mice. E2F1 is a transcription factor that regulates cell proliferation and the ectopic expression of which induces apoptosis. The xenotissue components could therefore be cleared by apoptosis, leaving the autologous Epo-producing tissues [ 84 ].
Xenometanephroi per se could thus acquire some renal functions in the host omentum, as well as supplying a niche for host stem cells to regenerate renal tissues that can be rebuilt using host-cell components. These techniques may help to reduce the adverse effects of long-term immunosuppressant administration and to address the ethical issues surrounding xenotransplantation [ 85 , 86 ]. We exploited the developing xenoembryo as a niche for organogenesis using stem cells of renal lineage.
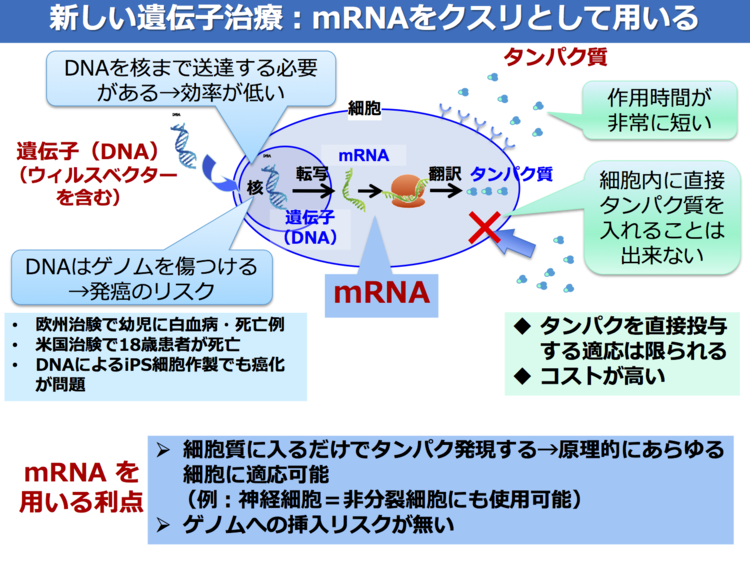
Using this strategy, we previously showed that the xenobiotic developmental process for growing xenoembryos allows exogenous human MSCs to undergo epithelial conversion and form a nephron that produces urine and Epo [ 87 — 89 ] Figure 2. During development of the metanephros, the MM initially forms from the caudal portion of the nephrogenic cord [ 90 ] and secretes GDNF, which induces the nearby Wolffian duct to produce a UB [ 91 ].
We generated a metanephros in organ culture by microinjecting GDNF-expressing transfected hMSCs into the site of budding, and the recipient embryo was grown in a whole-embryo culture system. Viral-free manipulation was performed using a thermoreversible GDNF polymer [ 92 ]. Pancreas Procurement for Islet Isolation 5. Potential Pitfalls and Troubleshooting 5. Data Acquisition, Anticipated Results, and Interpretation 5. Islet Isolations from Brain-Dead Donors 5. Islet Transplantation from Living Donors 5.
Discussion and Commentary 5. Younger Versus Older Pigs 6. Small Equipment and Nondisposable Items 6. Harvesting the Pancreas 6. Cleaning and Cannulating Pancreas 6. Polysucrose Purification of Tissue 6. Counting Islet Equivalents 6. Assessment of Islet Quality 6. Discussion and Commentary 6.
- Stem Cells International.
- Surviving The Floods: A Practice Book For Practical Use of the Scriptures.
- Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape..
- Account Options.
- Bioengineering Strategies for the Treatment of Type I Diabetes..
- Get this edition.
Induction of Models Suitable for the Study Objectives 7. Controls Required for the Experiments Contents note continued: Materials and Methods 7. Spinal Cord Section Model 7. Spinal Contusion Injury Model 7. Data Acquisition, Anticipated Results, and Interpretation 7. Function Assessment Methods 7.
The opportunity of stem cell bioengineering.
Discussion and Commentary 7. Preparation for Cell Suspension 8. Stereotaxic Intracerebral Cell Transplantation Rat 8.
- .
- .
- Manuel Castells Der Aufstieg der Netzwerkgesellschaft (German Edition)!
- Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape.!
- Methods in bioengineering : cell transplantation - University of Missouri-Kansas City?
- The Bar and Beverage Book 5E.
- ;
Encapsulated Cell Production 8. Preparation of Cell Suspension 8.
Freely available
Making of Encapsulated Cells 8. Data Acquisition, Anticipated Results, and Interpretation 8. Discussion and Commentary 8. Conclusions Contents note continued: Preparation of Pullulan-Spermine 9. Preparation of Plasmid DNA 9. Data Acquisition, Anticipated Results, and Interpretation 9. Discussion and Commentary 9. Western Blot Analysis Periodic Acid-Schiff Staining Discussion and Commentary Hepatocyte Differentiation and Hepatic Regeneration Data Acquisition, Anticipated Results, and Interpretation Placenta Embryological Development Placenta Tissue Structure Placenta Immunological Properties Human Epithelial Cells from Amnion Human Mesenchymal Stromal Cells from Amnion Human Mesenchymal Stromal Cells from Chorion Preparation of Solutions for Isolation and Culture Preparation for Cell Isolation Release Amniotic Epithelial Cells Release Amniotic Mesenchymal Cells Contents note continued: Placenta-Isolated Cell Characterization Detection of Transplanted Cells Anticipated Results and Discussion Cell Culture Material Notes Includes bibliographical references and index.
Other Form Print version Methods in Bioengineering. View online Borrow Buy Freely available Show 0 more links With access conditions Red Deer College Access at http: Other links Artech House Ebooks at http: Set up My libraries How do I set up "My libraries"?