He used a pinhole collimation and small detector size to project the distribution of gamma rays onto the scintillation screen [ 7 ]. Initially, the camera was used to scan patients administered by therapeutic doses of I. Disadvantages of this prototype were 1 small field of view of the imaging system 4 inch in diameter and 2 poor image quality unless a high injected dose or long exposure time are applied.
In , Anger succeeded in developing the first efficient scintillation camera, and marked progress in the detection efficiency was realized by using an NaI Tl crystal, photomultiplier PMT tubes, and a larger field of view. Spatial resolution and detection sensitivity are two important performance characteristics that play an important role in molecular imaging research using SPECT and PET tracers. Many factors serve to impact the final reconstructed images of data acquired from a PET scanner. These are crystal size, positron range, photon acollinearity, intercrystal interaction and scatter, depth of interaction and the reconstruction algorithm.
- An Historical Account of the Black Empire of Hayti.
- The Leading Facts of English History;
- Onthou jy nog die liefde? (Afrikaans Edition)!
In preclinical PET machines, positron range appears to be the most important challenge that needs to be tackled to improve the spatial resolution of the PET images. However, the current generation of clinical PET scanners is slightly affected by the positron range, but correction of the phenomenon was shown to be effective in positron emitters of high maximum kinetic energy [ 10 — 12 ].
The gamma camera relies on hardware collimators to determine the photons trajectory and hence able to localize the emission site by analyzing the electronic signal detected by the imaging detector. This hardware collimation plays a significant role in reducing the overall system sensitivity as well as the spatial resolution. However, a new trend of designing semiconductor systems is emerging in the field, providing a significant improvement in spatial resolution, and other performance measures [ 13 , 14 ]. SPECT degrading factors have been extensively studied in the literature and, namely, include attenuation, scatter and resolution effects, in addition to motion artifacts.
Nevertheless, correction for photon attenuation, scatter and partial volume would collectively improve the detection and estimation task [ 15 ]. This is particularly important for small structures and in small energy radionuclides such as I [ 16 ]. Unlike PET, single photon emitting radiopharmaceuticals have several features in the context of molecular imaging such as cost and wide availability of the radioligands as well as relative ease of labeling. Small animal imaging using preclinical scanners and PET radiopharmaceuticals showed better capability in tracer kinetic studies when compared to its SPECT counterparts.
PET compounds have been extensively used in compartmental modeling and kinetic analysis. The early work done on small animal imaging using SPECT tracer was to use a gamma camera equipped with pinhole collimator s of very small aperture size. Although parallel hole is the most commonly used collimator in many nuclear medicine procedures, pinhole imaging has a well-recognized role particularly for small organs such as thyroid and parathyroid imaging. In bone joints as well as in some pediatric studies, pinhole can also improve the spatial resolution by magnifying small structures of different tracer uptakes.
In recent years, pinhole geometry was found an increasing interest in designing SPECT scanners with superb spatial resolution and this has been attained by minimizing the aperture size to sub-millimeter range and specialized collimator geometry. However, the cost paid for this improved spatial resolution is a reduction of the detection efficiency.
The later was partially tackled by increasing the number of holes for improvement of count collection and statistical quality. Pinhole geometry is not similar to parallel hole geometry where one-to-one magnification is achieved. The geometric magnification provided by pinhole geometry is a function of the object distance from the aperture as well as distance of the aperture from the detector surface in addition to the effective aperture diameter. Nothing is free, this takes place with a reduction of the imaging field of view. Another problem encountered when pinhole collimator is used in tomographic acquisition is data insufficiency and the resulting images could suffer from reconstruction errors.
Image reconstruction using iterative techniques have solved many problems that were not possible to achieve with analytic approaches. In clinical and preclinical arena involving both SPECT and PET imaging, iterative reconstructions were found superior to analytic approaches in many aspects of diagnostic quality and quantitative accuracy. Besides its treatment to image noise, iterative reconstruction for pinhole geometry can correct for photon attenuation, scatter, and system response function.
Edge penetration and parallax errors can also be modeled in the reconstruction scheme reducing the blurring effect allowing for enhanced spatial resolution [ 17 ].
International Journal of Molecular Imaging
A conventional gamma camera can be used by manufacturing pinhole collimators of very small aperture size. It provides large field of view such that better magnification can be achieved. The other alternative is to use pixilated detectors that have better intrinsic properties or semiconductor detectors that fit with the resolution requirements of small animal imaging and, meanwhile, better than that provided by the conventional gamma camera. Regardless of their cost, semiconductor detectors are more compact and allow for system portability and can be manufactured in pixilated structure providing better spatial resolution.
Using a large field of view gamma camera serves to improve the magnification by providing large projection area onto the detector surface for the subject under investigation. However, some recent scanners are implementing pixilated detectors that have an intrinsic resolution equivalent to the segmentation size. This, to some extent, obviates the need to use detector width of size equivalent to the standard clinical gamma camera [ 5 ].
Thallium-activated sodium iodide NaI Tl crystal is the conventional scintillator used in most clinical designs. For example, Funk et al. The system showed submillimeter spatial resolution and high detection efficiency when compared to dual head gamma camera, permitting shortened acquisition time and a reduced injected dose. Several designs were proposed for pinhole geometry, including rotating gamma camera, stationary detector but rotating collimators, or completely stationary camera [ 19 ].
The collimator is cylindrical in shape with relatively large number of pinholes i. A resolution of 0. The values are less in case of rat imaging 0. The Inveon is another commercial design provided by Siemens Medical Solutions.
Molecular SPECT Imaging: An Overview
The heads can be equipped with various parallel-hole, single- or multipinhole collimators, including mouse general body as well as mouse brain imaging with possible submillimeter spatial resolution. It provides opportunities to scan individual organs or whole body images. The SPECT system accommodates single and multiple pinhole collimators as well as parallel hole collimators to address a broad range of study needs.
It can be configured to have 1,2,3 or 4 CZT cameras providing a variety of spatial resolution, detection sensitivity, and scanning field of view [ 20 ]. The Bioscan system has a four-detector head that consist of NaI Tl crystal. It uses a patented multiplexed-multipinhole collimator design that can reach 36 pinholes or eve more. They produce high resolution anatomical images in addition to generating a subject-specific attenuation map able to correct for photon attenuation.
MRI machines can have a better soft tissue contrast, not relying on ionizing radiation, and provide high spatial resolution as mentioned earlier. However, new photodiodes that are less prone to magnetic fields can be very helpful in such designs. Molecular imaging is an emerging field of study that deals with imaging of disease on a cellular or genetic level rather than on a gross level [ 27 ]. With the emergence of the new field of molecular imaging, there is an increasing demand for developing sensitive and specific novel imaging agents that can rapidly be translated from small animal models into patients.
SPECT and PET imaging techniques have the ability to detect and serially monitor a variety of biological and pathophysiological processes, usually with tracer quantities of radiolabeled peptides, drugs, and other molecules at doses free of pharmacologic side effects [ 28 ]. RIMPs are highly specific radiolabeled imaging agents used to visualize, characterize, and measure biological processes in living systems. Both, endogenous molecules and exogenous probes can be molecular imaging agents. In the design and development of an ideal RMIP, it is important to identify first a molecular imaging probe MIP , which may be a biochemical or a synthetic molecule, specific for a biological process such as metabolism, angiogenesis, and apoptosis or a molecular target such as hexokinase, thymidine kinase, and neuroreceptor in an organ, or tissue of interest.
Radiolabeling of RMIPs Generally, the radiolabeling process of molecular imaging agents can be categorized as follows. Isotope Exchange Where the preparation is obtained by direct exchange of stable atom s of an element in a molecule with one or more nuclide of a radioisotope of the same element. Metal Chelation In this method, a chelating agent radiometal such as 99m Tc and In is being introduced into an organic compound producing a ligand with different biological and chemical features than both the conjugated two partners. Certain peptides and monoclonal antibodies can successfully be labeled by the metal chelation procedure but only in the presence of a bifunctional chelate BFC by conjugation with the peptide or protein first and then bind the radiometal to the BFC conjugated molecule.
Classification of RMIPs Based on their clinical utility and the nature of application for which they are designed as tools in the drug development program, four classes of RMIPs have been identified [ 30 ]. Since the advent of hybridoma technology for production of MoAbs in [ 32 ] which was designed originally as an in vivo tumor localizing agents, only few have reached a point of proven clinical utility [ 33 ]. Labeling of MAbs can be accomplished with several radionuclides, among which In, Tcm, I, I, and I, where most of them are commonly used in nuclear medicine [ 34 ] and listed in Table 1.
A number of monoclonal labeled antibodies using In or Y radionuclides are described in Table 2. CEA is expressed in a variety of carcinomas, particularly of the gastrointestinal tract GIT and can be detected in the serum. It is a single-dose kit introduced by Immunomedics Europe in In vitro assays have been developed that use Annexin V to detect apoptosis in hematopoietic cells, neurons, fibroblasts, endothelial cells, smooth muscle cells, carcinomas, and lymphomas. This can be mitigated by peptides whose molecular size is smaller than that of proteins and where the peptidases can act for rapid excretion by degradation of the peptides.
Peptides have been labeled with In and 99m Tc in the same manner of the monoclonal antibodies. Some useful labeled compounds are listed in Table 3. Metabolic imaging can be achieved using natural or exogenous radiolabeled substrates which participate in the particular metabolic process. The design of such tracers is based on the physiological concepts such as turnover of oxygen, glucose, amino acids, fatty acids, or DNA precursors. Commonly, I and 99m Tc derivatives are used as SPECT tracers for this function, however, the obvious chemical changes occur with this conjugation which can alter the physiological properties of the tracer module limits their application in molecular imaging.
Radiolabeled amino acids pass the blood-brain barrier and are accumulated in tissues via a specific amino acid transport system [ 54 ]. In analogy with PET, IMT is not incorporated into proteins [ 55 ] and its uptake reflects amino acid transport [ 56 ]. As thymidine kinase 1 tk1 shows an S-phase dependant expression, the intracellular accumulation of labeled nucleosides that are substrates for TK1 reflects DNA synthesis and thus tumor proliferation.
I[ 57 ] and I[ 58 ] labeled 2-arabino-fluroiododeoxyuridine FIAU has been used successfully as nucleoside analog for tumor proliferation detection. Nucleoside derivatives that are selective substrates for herpes simplex virus thymidine kinase HSVtk have been developed for the in vivo visualization of transgene expression using the HSVtk gene as a reporter gene.
Radioiodine labeled uracil compounds e. When otherwise healthy tissues lose their O 2 supply acutely, the cells usually die, whereas when cells gradually become hypoxic, they adapt by upregulating the production of numerous proteins that promote their survival. These proteins slow the rate of growth, switch the mitochondria to glycolysis, stimulate growth of new vasculature, inhibit apoptosis, and promote metastatic spread [ 59 ].
Most hypoxia markers contain a nitroimidazole moiety as a reactive chemical species. Nitroimidazoles can be used as probes to detect hypoxia as they are reduced intracellularly in all cells, but in absence of adequate supply of O 2 , they undergo further reduction to more reactive products which bind to cell components and are finally trapped in the hypoxic tissue [ 60 ].
Iodine labeled iodoazomycin arabinoside IAZA has been validated in animal model in the preclinical phase [ 62 ], but no clinical studies with this agent have been reported so far. In blood cell labeling, the plasma transferrin competes for the In and reduces the labeling efficiency because In binds with higher efficiency to transferrin than blood cells, and, therefore, isolation of the desired blood component from plasma permits easy labeling of either platelets or WBCs.
Stabilization of the 99m Tc-HMPAO primary complex is required due to the high degradation rate of its radiochemical purity. This could be achieved by adding stabilizers like methylene blue in phosphate buffer or cobalt II -chloride to the reaction vials [ 64 ], however, these reagents should not be used when the complex formulation is designed for labeling of leukocytes. Ioflupane binds specifically to structures of the nerve cells ending in the striatum area of the brain that are responsible for the transportation of dopamine.
This binding can be detected using tomographic imaging [ 67 ]. The application for imaging modalities in preclinical models is highly valuable as it has a great scope for noninvasively studying dynamic biological processes at the molecular and cellular level. This technology plays a key role in bridging bench studies of disease modelled in vitro to their implementation in clinically relevant animal models of diagnostic or therapeutics for their translation into the clinics.
In fact, the implementation of imaging in rodents has a great relevance because of the widespread use of genetically modified mice in biomedical research and the need to characterise the in vivo anatomical and functional phenotypes of animal disease models. Another advantage of imaging modalities developed for small animals is that the technology can relatively be translated directly for application to clinical practice. Preclinical SPECT systems have a great scope of applications in cardiovascular research, including the study of myocardial functions e.
Cardiac and respiratory motion is one of the major challenges when imaging rodent mouse heart rate: Gated acquisition is therefore required to minimise any movement artefacts. Another key area is the visualization of necrotic tissues and related tracers during myocardial infarction MI. Some studies have assessed myocardial ischemia in rat heart models after left coronary artery occlusion by using 99m Tc-glucarate [ 74 ]. In vivo visualization of necrosis may help to detect MI at early stages and may provide a good approach for evaluating the antinecrotic effect of developing drugs for ischemic heart disease.
On the same vein, the visualization of apoptotic cell death is another important target for non-invasive imaging [ 75 ]. Hence, the development of tracers e. Imaging such a mechanism is important for the understanding of infarct healing and post-MI remodelling. Another relevant area of cardiovascular imaging is the development of methods to characterise the formation and prognosis of atherosclerotic plaques. Plaque rupture results in severe cardiac events including MI and sudden death, hence, there is an important need for developing tools that can assist in predicting the plaques vulnerability to rupture.
Not all the plaques carry the same risk, and the criteria for imaging their vulnerability relies on the detection of inflammatory cell infiltration, platelet aggregation, tissue matrix degradation, large lipid contents and apoptosis. In many other cases, however, comparative inferences are desired such as parametric studies of the influence of task difficulty on a cognitive process, and thus control of such factors as learning, adaptation, and salience must be considered.
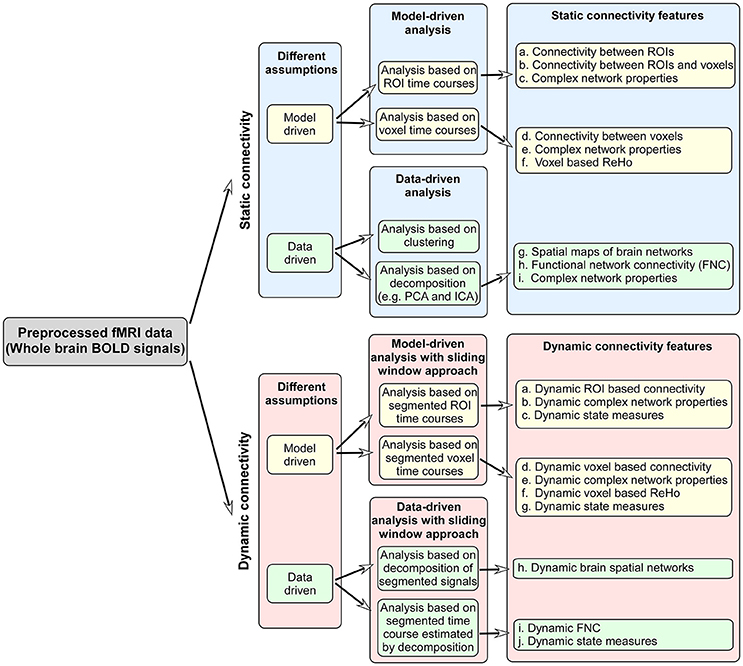
Once the images have been acquired, the time series data must be processed to obtain maps of brain activation. The noise results from thermal sources in the subject and electronics, bulk motion of the head, cardiac and respiratory-induced noise, and variations in baseline neural metabolism. Because the noise can sometimes be larger than the signal of interest, fMRI analyses compare the signal difference between the states using a statistical test. These tests result in an activation map that is a function of the probability that the brain states differ.
The statistical test for activation can utilize a general linear model GLM 27 , 63 , cross-correlation with a modeled regressor 2 , or one of several data-driven approaches such as independent components analysis ICA In all cases, the activation testing is preceded by a series of preprocessing steps. The steps in pre-processing can include all or some of the following: The analysis of fMRI data continues to be a subject of intense research at this time, and is one about which numerous books have been written, to which the reader is referred for further information e.
Resolution in fMRI is limited primarily by SNR because of the necessity for rapid acquisition of time series information. Thus, as T acq is reduced for single shot imaging typically 20—30ms the pixel size must be increased over that for conventional anatomic imaging to maintain an acceptable SNR. Accordingly, the typical fMRI pixel size is 3—4 mm, although with higher field magnets 7T a pixel size of microns or less may be readily achieved NIRS resolution is low 10—20 mm and limited predominantly by the strong scatter and attenuation of IR photons which also limits the depth of cortex that can be imaged within a banana-shaped region connecting optodes , the modest density of optodes and the ill-conditioned inverse problem of reconstructing 3D maps of [Hb] from scalp recordings This is much slower than the underlying neural processes, and temporal information is thereby heavily blurred.
Nevertheless, by jittering event-related stimuli and using appropriate analysis methods 11 temporal inferences in the ms resolution range can be achieved PET scans require minutes to complete because of the low count rates of injected radio nuclides, so changes in neural processes can only be studied by repeated scanning.
EEG and MEG, on the other hand, have millisecond temporal resolution and can easily capture the dynamics of evoked responses that last a few ms to several hundred ms. Multimodal approaches combining fMRI and EEG use fMRI maps as spatial priors to reconstruct high temporal resolution electrophysiology, thereby gaining resolution in both dimensions From the discussion above, a primary strength of fMRI is its relatively high spatial resolution and availability. In addition, it is readily available to both clinical and academic researchers, is noninvasive, and can provide high resolution anatomic scans in the same session to use for localization, vessel identification, 45 or development of maps of white matter connectivity through the use of diffusion tensor imaging DTI 5.
Because BOLD contrast derives from the sluggish hemodynamic response to metabolic changes, a significant weakness is its low temporal resolution. Many methods have been developed to diminish these susceptibility losses, although most involve some tradeoff of SNR in magnetically uniform brain regions 18 , 19 , 31 , 34 , 58 , 60 , Finally, the high magnetic fields require customized stimulus delivery and subject response systems, again limiting flexibility and complicating multimodal experiments such as concurrent EEG recording.
Over the years, a number of investigators have attempted to develop alternatives to BOLD contrast using direct neural current detection 9 , although by now it is understood 42 that the weak size of the neural current signal relative to physiological noise makes a breakthrough unlikely. Another alternative is the use of diffusion weighted imaging to demonstrate activation-related changes in populations of bound vs. A potential advantage is that such diffusion related changes may have more rapid responses than BOLD methods.
However, again the signals are weaker than BOLD contrast and their biophysical origin is still unclear While a modest research effort will continue in improving acquisition technology, the bulk of research in the development of fMRI has shifted to its application to answering more complex questions in cognitive neuroscience. One promising area is that of using activation maps as input to classification and state change algorithms to predict or classify cognitive behavior, such as predicting brain states 44 , 53 also see, e. Norman for a review Other emerging uses of fMRI include the development of quantitative measures, i.
A cautionary note, however, is that because of the small BOLD responses typical of cognitive processes, most studies are limited to employing group statistics to make inferences about populations rather than about individuals. Finally, feedback derived from real-time fMRI has been shown to allow subjects to learn pain-reduction strategies 24 , enhance sensorimotor control 23 and to control relevant brain regions in mood disorder experiments The reader is also referred to Bandettini 1 for additional considerations regarding the future of fMRI.
Informed by fMRI, more sophisticated modeling of brain networks is certain to lead to new levels of understanding of the human brain. The author has no conflicts to declare. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
National Center for Biotechnology Information , U. Neurosurg Clin N Am. Author manuscript; available in PMC Apr 1. Author information Copyright and License information Disclaimer. The publisher's final edited version of this article is available at Neurosurg Clin N Am. See other articles in PMC that cite the published article. Synopsis Blood Oxygen Level Dependent BOLD functional magnetic resonance imaging fMRI depicts changes in deoxyhemoglobin concentration consequent to task-induced or spontaneous modulation of neural metabolism. Introduction Functional Magnetic Resonance Imaging fMRI is a class of imaging methods developed in order to demonstrate regional, time-varying changes in brain metabolism 3 , 37 , Basis for fMRI fMRI is of course based on MRI, which in turn uses Nuclear Magnetic Resonance coupled with gradients in magnetic field 38 to create images that can incorporate many different types of contrast such as T1 weighting, T2 weighting, susceptibility, flow, etc.
Open in a separate window. The fMRI experiment The typical fMRI task activation experiment utilizes visual, auditory or other stimuli to alternately induce two or more different cognitive states in the subject, while collecting MRI volumes continuously as described above.
Analysis methods Once the images have been acquired, the time series data must be processed to obtain maps of brain activation.
- Neuroimaging.
- Overview of Functional Magnetic Resonance Imaging.
- Overview of Functional Magnetic Resonance Imaging;
- Molecular SPECT Imaging: An Overview?
Comparisons with other functional imaging modalities fMRI can be compared to other imaging methods used to obtained functional assessment of brain metabolism in terms of spatial and temporal resolution and availability. Strengths and weaknesses of fMRI From the discussion above, a primary strength of fMRI is its relatively high spatial resolution and availability.
Navigation menu
Acknowledgments The author is indebted to C. Chang for suggestions on the manuscript.

Footnotes The author has no conflicts to declare Publisher's Disclaimer: Ann N Y Acad Sci. Processing strategies for time-course data sets in functional MRI of the human brain. Time course EPI of human brain function during task activation. Differential age effects on cerebral blood flow and BOLD response to encoding: Functional mapping of the human visual cortex by magnetic resonance imaging. Handbook of MRI Pulse sequences. The respiration response function: Bodurka J, Bandettini PA. Toward direct mapping of neuronal activity: MRI detection of ultraweak, transient magnetic field changes.
The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Buxton R, Frank L. A model for the coupling between cerebral blood flow and oxyen metabolism during neural stimulation.
J Cereb Blood Flow Metab.
There was a problem providing the content you requested
Dynamics of blood flow and oxygenation changes during brain activation: Magnetic Resonance in Medicine. A method for making group inferences from functional MRI data using independent component analysis. Influence of heart rate on the BOLD signal: Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Reduction of susceptibility artifact in gradient-echo imaging. Constable R, Spencer D. Composite image formation in Z-shimmed functional MR imaging. Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities.
Learned regulation of spatially localized brain activation using real-time fMRI. Control over brain activation and pain learned by using real-time functional MRI.