It is FDA-approved for the treatment of myeloma that has relapsed after two prior treatments or where resistance has developed following the last treatment. It was also found to induce high quality responses as third line salvage therapy with acceptable toxicity in a significant proportion of homogeneously pre-treated myeloma patients with progressive disease after autologous transplantation and thalidomide. In a Phase 3 trial involving myeloma patients treated with at least one prior therapy, bortezomib increased median, improved overall survival, and increased response rate, compared with high-dose dexamethasone [ ].
In combination with doxorubicin and gemcitabine, bortezomib was also found to be effective in heavily pretreated, advanced Cutaneous T cell Lymphomas CTCL [ ]. On the contrary, the use of bortezomib was discouraged after a phase II study revealed that found in combination with dexamethasone, bortezomib is not active in heavily pre-treated patients with relapsed Hodgkin's lymphoma [ , ]. Possesses immunomodulatory, anti-inflammatory, and anti-angiogenic properties, although the precise mechanisms of action are not fully understood. Thalidomide was the first angiogenesis inhibitor to demonstrate clinical efficacy in multiple myeloma [ 37 , ].
Specifically in myeloma, thalidomide down-regulated VEGF secretion from bone marrow endothelial cells obtained from patients with active disease. In a landmark Phase 2 clinical trial, previously treated patients with refractory myeloma received thalidomide monotherapy [ ]. These results led to many subsequent clinical studies of thalidomide in myeloma, leading ultimately to FDA approval of the drug in , for the treatment of newly diagnosed multiple myeloma, in combination with dexamethasone.
Join Kobo & start eReading today
Long-term outcome measures, including time-to-progression TTP and PFS, were recently reported for a patient randomized, placebo-controlled Phase 3 clinical trial of a similar protocol in newly diagnosed multiple myeloma, with comparable overall response rates [ ]. Significant increases resulted in both median TTP and median PFS for the thalidomide plus dexamethasone group versus dexamethasone alone.
Thalidomide was found to be moderately tolerated and minimally effective in patients with histologically proven advanced hepatocellular carcinoma [ ]. Thalidomide provided no survival benefit for patients with multiple, large, or midbrain metastases when combined with WBRT whole-brain radiation therapy [ ]. On the contrary, thalidomide did not significantly add to the efficacy of the fludarabine, carboplatin, and topotecan FCT regimen in poor prognosis AML patients [ ] and was also ineffective in improving prognosis or decreasing plasma VEGF levels in patients with persistent or recurrent leiomyosarcoma of the uterus [ ].
While conventional anti-angiogenic therapy is based on Maximum Tolerated Doses MTD , the cells involved in angiogenesis may regenerate during the three- to four-week interval between cycles of the chemotherapy. Taking advantage of the fact that endothelial cells are about 10— times more susceptible to chemotherapeutic agents than cancer cells, therapy based on daily, oral, low-dose chemotherapeutic drugs was designed.
Metronomic chemotherapy refers to the close, rhythmic administration of low doses of cytotoxic drugs, with minimal or no drug-free breaks, over prolonged periods. Metronomic therapy appears promising mainly due to the fact that its anti-angiogenic and anti-tumorigenic effects are accompanied by low toxicity, limited side effects, no need for hospitalization and allowing for feasible combinations with selective inhibitors of angiogenesis. There are several foreseeable advantages and opportunities for metronomic chemotherapy: In a pilot phase II study conducted by Correale et al [ ] to investigate the toxicity and activity of the novel metronomic regimen of weekly cisplatin and oral etoposide in high-risk patients with NSCLC, the objective response rate was Pharmacokinetic analysis showed that this regimen allowed a greater median monthly area under the curve of the drugs than conventional schedules.
In a Phase I trial of metronomic dosing of docetaxel and thalidomide, of the 26 patients with advanced tumors enrolled, prolonged freedom from disease progression was observed in Circulating endothelial progenitor cells EPCs also participate in tumor angiogenesis. In a study comparing the effects of metronomic chemotherapy over conventional dose-dense chemotherapy, it was found that the numbers of circulating EPCs and the plasma levels of VEGF increased sharply, doubling pre-therapeutic levels at day 21 after conventional chemotherapy, whereas under low-dose metronomic chemotherapy, the numbers of circulating EPCs decreased significantly and VEGF plasma concentrations remained unchanged.
These observations provide evidence that conventional dose-dense chemotherapy leads to rebound EPC mobilization even when given with adjuvant intention, while low-dose metronomic scheduling of cytotoxic substances such as trofosfamide may sharply reduce EPC release into the circulation. Combined bevacizumab and metronomic oral cyclophosphamide was also discovered to be a safe and effective regimen for heavily pre-treated ovarian cancer patients [ ].
Treatment with metronomic capecitabine and cyclophosphamide in combination with bevacizumab was shown to be effective in advanced breast cancer and additionally was minimally toxic [ ]. Metronomic treatment with carboplatin and vincristine associated with fluvastatin and thalidomide significantly increased survival of pediatric brain stem tumor patients. Tumor volume showed a significant reduction accompanied by increased quality of life [ ]. Thus, given the fact that the most evident effect of selective anti-angiogenic agents i.
Overall, metronomic chemotherapy was able to induce tumor stabilization and prolong the duration of clinical benefit, without much associated toxicity. Emerging evidence suggests that metronomic chemotherapy could also activate the host immune system and potentially induce tumor dormancy [ - ].
While angiogenesis as a hallmark of tumor development and metastasis is now a validated target for cancer treatment, the overall benefits of anti-angiogenic drugs from the perspective of impacting survival have left much to desire, endorsing a need for developing more effective therapeutic regimens e. There are now several agents that target the tumor vasculature through different pathways, either by inhibiting formation of the tumor neovasculature or by directly targeting the mature tumor vessels.
The main body of evolving evidence suggests that their effects are compounded by their synergistic use with conventional chemotherapy rather than individual agents. Anti-angiogenic drugs such as bevacizumab can bring about a transient functional normalization of the tumor vasculature. But long term inhibition of angiogenesis reduces tumor uptake of co-administered chemotherapeutic agents.
This underscores the need for discovering new targets for anti-angiogenic therapy in order to effectively prohibit angiogenesis and circumvent mechanisms that contribute to resistance mechanisms that emerge with long term use of anti-angiogenic therapies. It also warrants a need to define reliable surrogate indicators of effectiveness of the anti-angiogenic therapy as well as dependable markers for identifying the patients who are most likely to benefit from the combination of anti-angiogenic therapy and conventional chemotherapy.
Several new frontiers are emerging. New advances in understanding endothelial cells, which constitute the tumor vasculature, towards developing antiangiogenic strategies are one of the important ones [ , ]. Novel cellular targets such as integrins and microRNAs and novel treatment options such as possible use of pharmaconutrients to modulate angiogenic pathways need careful testing and evaluation [ - ].
Finally, the administration of these drugs in a metronomic schedule is likely to improve the overall response to anti-angiogenic drugs making it feasible to administer them with conventionally toxic chemotherapeutic drugs, thus increasing the armamentarium of drug combinations that can be employed for treatment. Research in the laboratory of R. National Center for Biotechnology Information , U. Journal List Oncotarget v. Published online Mar 7. Samant and Lalita A. Author information Article notes Copyright and License information Disclaimer.
Received Feb 28; Accepted Mar 7. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This article has been cited by other articles in PMC. Abstract Since angiogenesis is critical for tumor growth and metastasis, anti-angiogenic treatment is a highly promising therapeutic approach.
Bestselling Series
The Activators Tumor cells activate signaling pathways that promote uncontrolled proliferation and survival. The Inhibitors If angiogenesis is so critical for the tumor growth, then agents that inhibit angiogenesis would have great therapeutic value. Based on functionality, the anti-angiogenic drugs can be sub-divided into three main groups: Open in a separate window.
Targets of FDA-approved angiogenesis inhibitors: Drugs that inhibit growth of endothelial cells e. Drugs that block angiogenesis signaling e. Drugs that block extracellular matrix breakdown e. There are currently seven approved anti-cancer therapies in two primary categories: Small molecule tyrosine kinase inhibitors TKIs of multiple pro-angiogenic growth factor receptors.
Since discussing all of them is beyond the scope of this article, we have focused our discussion on the three TKIs that are currently approved as anti-cancer therapies: Acknowledgments Research in the laboratory of R. Development and differentiation of endothelium. Angiogenesis is coming of age. Burri PH, Djonov V.
Intussusceptive angiogenesis--the alternative to capillary sprouting. Its emergence, its characteristics, and its significance. Interactions between cancer cells and the microvasculature: A rate-regulator for metastasis. J Cancer Res Clin Oncol. Factors influencing the neovascularization of experimental tumours.
Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. Kos M, Dabrowski A.
- Still the Happiest People.
- I Missed the Spring.
- Violin Concerto in A minor, Op. 53, Movmt. 2, Adagio ma non troppo.
- Jews and Muslims in the Arab World: Haunted by Pasts Real and Imagined?
- Capabilities.
Tumour's angiogenesis--the function of vegf and bfgf in colorectal cancer. Role of angiogenesis in tumor growth and metastasis. A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Id genes mediate tumor reinitiation during breast cancer lung metastasis. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth.
Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Expanding therapeutic targets in bladder cancer: Pseudo-active sites of protease domains: The hedgehog pathway transcription factor gli1 promotes malignant behavior of cancer cells by up-regulating osteopontin. Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis.
Dnajb6 induces degradation of beta-catenin and causes partial reversal of mesenchymal phenotype. Cox-2 as a target for cancer chemotherapy. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Mignatti P, Rifkin DB. Plasminogen activators and matrix metalloproteinases in angiogenesis. Adhesive and proteolytic phenotype of migrating endothelial cells induced by thymosin beta Ann N Y Acad Sci.
Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Madhusudan S, Harris AL. Drug inhibition of angiogenesis. Targeting angiogenesis with integrative cancer therapies. The implications of angiogenesis for the biology and therapy of cancer metastasis. Ruegg C, Mutter N. Anti-angiogenic therapies in cancer: Achievements and open questions. Perspectives for medical, surgical and radiation oncology. Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Thalidomide is an inhibitor of angiogenesis. Selective targeting of the tumour vasculature. Phase ii study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer.
Bevacizumab for the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. Bevacizumab in combination with chemotherapy: First-line treatment of patients with metastatic colorectal cancer. Clinical benefit of new targeted agents in phase i trials in patients with advanced colorectal cancer. Sachdev JC, Jahanzeb M. Evolution of bevacizumab-based therapy in the management of breast cancer.
Clinical and biological activity. Bevacizumab and irinotecan in the treatment of recurrent malignant gliomas. How avastin potentiates chemotherapeutic drugs: Action and reaction in antiangiogenic therapy. Antiangiogenesis in haematological malignancies.
Angiogenic implications for signal transduction therapy of solid tumors. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: A single centre phase ii trial. A clinical practice setting, multicenter experience. Phase i study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase.
Merlano M, Occelli M. Review of cetuximab in the treatment of squamous cell carcinoma of the head and neck. Ther Clin Risk Manag. Phase i studies of anti-epidermal growth factor receptor chimeric antibody c alone and in combination with cisplatin. Clin J Oncol Nurs. Fda drug approval summary: Food and drug administration approval: Panitumumab for epidermal growth factor receptor-expressing metastatic colorectal carcinoma with progression following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens.
Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Open-label phase iii trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Wainberg Z, Hecht JR.
Panitumumab in colon cancer: A review and summary of ongoing trials. Expert Opin Biol Ther. Effects of palifermin on antitumor activity of chemotherapeutic and biological agents in human head and neck and colorectal carcinoma xenograft models. Augmentation of radiation response by panitumumab in models of upper aerodigestive tract cancer. A testing algorithm for determination of her2 status in patients with breast cancer.
Ann Clin Lab Sci. Nahta R, Esteva FJ. Her-2 breast assay, linked to herceptin, wins fda's okay. Monoclonal antibody approved for metastatic breast cancer Oncology Williston Park ; Response of metastatic breast cancer to trastuzumab?
- The Tempter Comes?
- The Slender Man.
- Journey of a Lifetime.
- MOTHER EARTH NEWS Digital Archive: 2011.
- Recent Advances in Anti-Angiogenic Therapy of Cancer.
- Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer.
A prospective, non-randomized phase-ii trial of trastuzumab and capecitabine in patients with her2 expressing advanced pancreatic cancer. Int J Gynaecol Obstet. Solamargine enhances her2 expression and increases the susceptibility of human lung cancer h and h69 cells to trastuzumab and epirubicin. Her2 expression in cervical cancer as a potential therapeutic target. Prognostic, predictive and therapeutic implications of her2 in invasive epithelial ovarian cancer.
Cp , nsc , osi , r Drugs R D. Herbst RS, Sandler A. A promising new approach to the treatment of advanced nsclc. Oncology Williston Park ; The first biologic in the management of pancreatic cancer. A phase ii trial of erlotinib in combination with bevacizumab in patients with metastatic breast cancer. Phase ii trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma.
Randomized phase ii study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. A phase ib dose-escalation study of erlotinib, capecitabine and oxaliplatin in metastatic colorectal cancer patients. Results from a monocentric phase ii trial of erlotinib in patients with metastatic prostate cancer. Phase i trial of erlotinib combined with cisplatin and radiotherapy for patients with locally advanced cervical squamous cell cancer. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer.
Nat Rev Drug Discov. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Sorafenib for the treatment of advanced renal cell carcinoma. Recent advances in the treatment of renal cell carcinoma and the role of targeted therapies. Food and drug administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma.
Sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Zhu AX, Raymond E. Early development of sunitinib in hepatocellular carcinoma. Acute hepatic failure following monotherapy with sunitinib for ovarian cancer. Major response to sunitinib sutene r in metastatic malignant phyllodes tumor of breast.
The current status and evolving role of sunitinib in non-small cell lung cancer. Furthermore, Benedito et al. Angiopoietin1 Ang1 and Ang2 bind Tie2, a tyrosine kinase receptor expressed in stalk and phalanx cells. Perivascular cell expression of Ang1 stabilizes and tightens the EC barrier by recruiting complexes between Tie2 and the phosphotyrosine vascular endothelial protein tyrosine phosphatase VE-PTP to cell-cell junctions and by preventing VEGFR2-induced internalization of the junctional molecule VE-cadherin In ischemic tissues, Ang1 promotes vessel growth and enlargement, but without inducing vessel leakage as VEGF does , making it a potential target for therapeutic angiogenesis EC-expressed Ang2 antagonizes Ang1 activity and thereby stimulates vessel destabilization and sensitizes ECs to proangiogenic signals Figure 1 and ref.
In this case, Tie2 translocates to cell-matrix contacts. However, Ang2 also stimulates angiogenesis by activating Tie2. Indeed, Ang2 attenuates Ang1-Tie2 activation in the presence of Ang1 in mature tumor supply vessels , but activates Tie2 signaling when Ang1 is absent in immature pericyte-deprived tumor vessels , which suggests that Ang2 is a partial agonist Ang2 also stimulates tip cell migration by activating integrins independently of Tie2 Figure 1 and ref.
Tie1, an orphan receptor homologous to Tie2, heterodimerizes with Tie2 and regulates Ang2 activity. In the presence of Tie1, Ang2 is unable to activate Tie2; however, loss of Tie1 reveals agonist capabilities of Ang2. Anti-Ang2 antibodies inhibit tumor angiogenesis and growth and improve the antiangiogenic efficacy of VEGF blockers in xenograft models 22 , while a combination of angiopoietin blockers and cytotoxic drugs increases the progression-free survival PFS of patients with ovarian cancer Simultaneous targeting of angiopoietins and VEGF by the chimeric decoy receptor double antiangiogenic protein DAAP also inhibits tumor angiogenesis and growth in preclinical models Nonetheless, the effects of Ang2 on tumor progression upon over- or underexpression are complex and often divergent ECs express guidance receptors that probe the environment Figure 2.
Nrp1, alone or complexed to plexin family members, interacts with semaphorins Sema. Robo4 is expressed in ECs, but its role remains debated, as it has been attributed both chemoattractant and repellant activity 29 , However, when binding to Unc5B, a netrin receptor, Robo4 increases vessel integrity and reduces angiogenesis by inhibiting VEGF signaling VEGF also promotes the expression of the transcription factor Hlx1, which increases expression of Unc5, plexin 5, and Sema3G, suggesting feedback with Robo4 Hlx1 is expressed in sprouting ECs, where it maintains the stalk cell phenotype by regulating repulsive signals Ephrins activate Eph receptor tyrosine kinases to regulate developmental vessel morphogenesis In zebrafish, angioblasts form a precursor vessel that segregates into discrete arterial and venous vessels.
Ephrin-B2—expressing ECs, fated to form arterial vessels, segregate from EphB4-expressing ECs, which become venous vessels due to repulsive cues Ephrin-B2 activates Eph receptors in a positive feedback loop and has its own reverse signaling activity, which is important for EC morphology and motility Furthermore, antibody blockade of Ephrin-B2 inhibits tumor angiogenesis in preclinical studies When tip cells of adjacent vessels meet via filopodia, they connect and anastomoze Figure 2.
Buy Guidance Molecules in Cancer and Tumor Angiogenesis - Microsoft Store
Imaging in zebrafish reveals that cell junctions at the site of contact expand into rings, generating an interface of apical membrane compartments In disease, macrophages have contextual effects. In ischemia they promote collateral vessel growth 42 , while in tumors M1-polarized macrophages are tumoricidal, but M2-polarized macrophages promote tumor vascularization by producing proangiogenic factors Targeting myeloid cells is gaining increasing attention for blocking tumor angiogenesis and growth Possible targets include placental growth factor PlGF , which promotes M2 polarization 45 , or Ang2, which increases macrophage association with tumor blood vessels 46 , The oxygen sensor HIF-prolyl hydroxylase domain protein 2 PHD2 also modulates the macrophage phenotype and regulates collateral vessel growth in ischemia Stalk cells elongate the sprout shaft Figure 2.
In vitro, Notch inhibits EC proliferation; however, stalk cells must proliferate to elongate the shaft in vivo. To overcome this, stalk cells express the Notch target Notch-regulated ankyrin repeat protein Nrarp , which limits Notch signaling at branch points while allowing continued Wnt signaling to promote EC proliferation and vessel stability Because of the pro—stalk cell activity of Notch, posttranslational modifications finely tune its activity to prevent excessive signaling. The NICD is acetylated, which stabilizes the protein against ubiquitylation-dependent proteasomal degradation.
Interestingly, sirtuin-1 is more active during fuel and energy stress, which suggests that it promotes vessel branching via Notch inactivation to guide ECs to fuel-rich areas How targeting these stalk cell signals can be used therapeutically for cancer remains to be determined. In agreement with this model, Alk1 inhibits retinal angiogenesis by cooperating with Notch: Mural pericytes reduce EC proliferation, migration, and vessel leakage, thereby stabilizing nascent vessels Figure 2 and refs.
Ang1 was previously suggested to promote pericyte coverage of blood vessels However, conditional global Ang1 gene inactivation studies showed that early Ang1 deficiency causes vascular morphogenesis defects, which are caused by cardiac defects and secondary flow disturbance, without affecting pericyte recruitment In postnatal angiogenic conditions, Ang1 deficiency accelerated angiogenesis, which suggests that Ang1 is dispensable for quiescent vessels but modulates the vascular response after injury Reduced pericyte coverage is associated with metastasis in patients, and overexpression of PDGF-B increases pericyte coverage that results in tumor growth inhibition.
Guidance Molecules in Cancer and Tumor Angiogenesis
Concerns have been raised regarding pericyte targeting, as this increases epithelial-to-mesenchymal transition and metastasis because of a reduced barrier for tumor cells to intravasate Phalanx ECs line quiescent vessels Figure 3. Once the hypoxic tissue is perfused by neovessels, levels of angiogenic signals are reduced, and proquiescent molecules are increased. Phalanx cells in a tightly apposed monolayer optimize conduction of blood flow, establish tissue barriers, and form intercellular junctions to tighten the EC barrier. An oxygen-sensing system ensures that ECs normalize abnormalities in structure and function of ECs to readapt oxygen supply to tissue needs.
It has been postulated that the molecular players and vascular branching model in pathological angiogenesis are parallel to developmental angiogenesis, but have dysregulated expression. However, some molecules have different functions during physiological and pathological angiogenesis. Stromal cell PlGF production, induced by contact with tumor cells, not only promotes angiogenesis in the leukemic bone marrow or medulloblastoma, but also stimulates tumor cell proliferation via Nrp1 signaling 74 , Although it is superfluous for vascular development, VEGF-B promotes contextual enlargement of myocardial capillaries 76 or growth of coronary vessels Another example is ataxia teleangectasia mutated ATM , which only regulates angiogenesis in disease, not in health These examples and others suggest that part of the molecular basis of pathological angiogenesis is different from that in vascular development.
Moreover, insights obtained from developmental angiogenesis models may not completely recapitulate the mechanisms that drive human pathological angiogenesis. To further our understanding of antiangiogenic medicines, it is therefore essential that sufficient feedback about the mechanism of pathological angiogenesis is provided by bedside-to-bench research.
Bevacizumab also shows efficacy in the neoadjuvant setting in breast cancer Despite the success of antiangiogenic drugs, several questions warrant further research to improve anticancer treatment. First, some cancers are resistant; even in responsive patients, antiangiogenic drugs generally prolong survival only in the order of months.
The FDA revoked the approval of bevacizumab for metastatic breast cancer In general, clinical efficacy is lower than that observed in preclinical cancer models These models often represent rapidly growing ectopic tumors that do not reflect the heterogeneous human cancers developing over years in situ. Even transgenic models do not fully reflect the multistep carcinogenesis that occurs in humans.
Another concern is that the majority of preclinical studies were undertaken in the neoadjuvant setting, which is a poor model for human metastatic cancer Moreover, many drug combinations that proved ineffective were not studied preclinically Tumor ECs engineered to overexpress DLL4 develop enlarged mature vessels that are resistant against VEGF blockade, while inhibition of Notch signaling restores the sensitivity to antiangiogenic drugs in a xenograft model PlGF and FGF2 plasma levels increased prior to progression of colorectal cancer patients treated with bevacizumab and chemotherapy Cancers also switch between different modes of vascularization, further explaining the resistance to VEGF blockade Figure 4.
Besides sprouting angiogenesis, they use vessel cooption by growing around preexisting vessels , vascular mimicry replacement of ECs by tumor cells , and vasculogenesis vessel growth from bone marrow—derived progenitor cells , although the clinical relevance of these mechanisms remains unclear 87 — For instance, metastases of melanoma and lung cancer grow in an angiogenesis-independent manner around existing vessels or switch to vessel cooption upon treatment with bevacizumab Furthermore, cancer stem cell—like cells differentiate to ECs that exhibit reduced sensitivity to VEGF blockade 88 , Resistance in certain cancers is associated with pericyte-covered vessels, while tortuous uncovered vessels are observed in primary resistance After development, the vasculature rarely extends, but does so in tumor formation.
Tumor vascularization occurs via a number of potential mechanisms. While angiogenesis is the most investigated, and the focus of this Review, other mechanisms have been observed. Endothelial progenitor cells EPCs , which can either reside in the vascular wall or migrate from bone marrow in response to chemoattractants from the tumor cell, can differentiate into ECs and contribute to vessel formation.
Vascular mimicry can also occur, whereby tumor cells can act as replacement cells for ECs. Another possibility is that chromosomal abnormalities in putative cancer stems cells allows tumor cells to differentiate into ECs. Other mechanisms by which tumor cells can obtain a blood flow include vessel cooption, whereby the tumor cell arises near to or migrates toward a preexisting blood vessel, or the process of intussusception, whereby a preformed vessel splits into two daughter vessels by the insertion of a tissue pillar.
Antiangiogenic drugs induce a systemic proinflammatory and proangiogenic burst in tumor-bearing healthy mice by upregulating PlGF, G-CSF, and osteopontin 95 , which induce mobilization of resistance-conferring MDSCs 96 , The tumor microenvironment can also cause refractoriness. For instance, pancreatic adenocarcinomas have high interstitial fluid pressure due to abundant deposition of hyaluronic acid, which impairs perfusion and drug distribution Notably, disaggregation of hyaluronic acid by enzymatic treatment improved perfusion Another hypothesis to explain the lower than expected efficacy of VEGF-targeted antiangiogenic drugs is that these treatments increase, rather than reduce, tumor malignancy.
Indeed, certain preclinical studies show enhanced metastasis in tumor-bearing mice treated with VEGF-blocking drugs, such as sunitinib 79 , 84 , 99 , However, these findings remain debated because other preclinical studies did not detect increased metastasis , , and large meta-analyses have not shown more metastatic dissemination in patients 79 , Strategies combining antiangiogenesis with inhibition of metastasis might be useful to increase therapeutic efficacy.
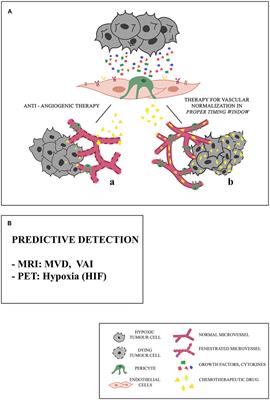
The precise mode of action of antiangiogenic drugs in cancer patients remains incompletely understood. Antiangiogenic drugs also inhibit VEGFR-expressing tumor cells 5 , or deprive cancer stem cells from EC-derived angiocrine signals Moreover, VEGF inhibitors reduce the number and self-renewal capability of cancer stem cells Alternatively, antiangiogenic drugs block excess VEGF levels secreted by tumor cells. This uncoordinated secretion of VEGF induces a chaotic proangiogenic response characterized by hyperbranching and lack of maturation, which renders tumor vessels dysfunctional.
Consequently, delivery of chemo- and radiotherapy is impaired. Nonetheless, vessel normalization can explain why bevacizumab works better when combined with chemotherapy. The challenge in the clinic is to identify agents that cause permanent tumor vessel normalization. Another question is the optimal duration and dose of antiangiogenic drug application.

Certain preclinical studies indicate rapid revascularization after cessation of treatment ; the clinical relevance of this observation requires future study. Adjuvant application of bevacizumab for 12 months had a beneficial effect on PFS of early-stage colorectal cancer patients when analyzed after 15 months, but this effect was lost after 36 months ; additionally, the outcome of patients with metastatic colorectal cancer was better when bevacizumab was given beyond progression A phase III trial in colon cancer showed a modest OS advantage for patients treated with bevacizumab beyond progression Bevacizumab beyond progression is currently being investigated in other cancer types.