You can choose from a wealth of available models and views, drop in a bunch of plugins and create the setup that fits you best. Many high-profile sites like the BBC iPlayer , people , magazines.
Check out some other sites which are using Catalyst. Use whatever templating language you like best: Template and many more are fully supported. Catalyst comes with its own lightweight test server for development. It automatically restarts when your sources have changed, so you get instant results! Don't re-invent the wheel! Use well-tested plug-ins for session management , user authentication , caching and much more. For more details and other platforms read the extended Installing Catalyst guide. Learn how to build your first complete Catalyst application with all the nuts'n'bolts of modern web applications.
This book by Kieren Diment, Matt Trout and other core Catalyst developers, published by Apress is now available for purchase from Amazon. Why a fresh Perl installation is a good idea: Plus, you will get the latest and greatest Perl version to work with. This guide shows you how to install the latest stable Perl version for different platforms alongside invaluable tools like local:: If you haven't installed cpanm and local:: This installs cpanm , local:: You can find more in-depth information about possible ways to install Catalyst on various platforms in the Installing Catalyst guide.
If you have any questions regarding the installation do not hesitate to swing by our IRC channel or send a mail to the Catalyst mailing list. Upon the addition of a small amount of manganese dioxide , the hydrogen peroxide reacts rapidly. This effect is readily seen by the effervescence of oxygen. Accordingly, manganese dioxide catalyses this reaction. Catalytic activity is not a kind of reaction rate, but a property of the catalyst under certain conditions, in relation to a specific chemical reaction. A catalyst may and usually will have different catalytic activity for distinct reactions.
See katal for an example. There are further derived SI units related to catalytic activity, see the above reference for details. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process regenerating the catalyst. The following is a typical reaction scheme, where C represents the catalyst, X and Y are reactants, and Z is the product of the reaction of X and Y:. Although the catalyst is consumed by reaction 1 , it is subsequently produced by reaction 4 , so it does not occur in the overall reaction equation:.
As a catalyst is regenerated in a reaction, often only small amounts are needed to increase the rate of the reaction. In practice, however, catalysts are sometimes consumed in secondary processes. The catalyst does usually appear in the rate equation.
Navigation menu
However [C] remains constant during the reaction so that the catalyzed reaction is pseudo-first order: As an example of a detailed mechanism at the microscopic level, in Danish researchers first revealed the sequence of events when oxygen and hydrogen combine on the surface of titanium dioxide TiO 2 , or titania to produce water. With a time-lapse series of scanning tunneling microscopy images, they determined the molecules undergo adsorption , dissociation and diffusion before reacting. The intermediate reaction states were: HO 2 , H 2 O 2 , then H 3 O 2 and the final reaction product water molecule dimers , after which the water molecule desorbs from the catalyst surface.
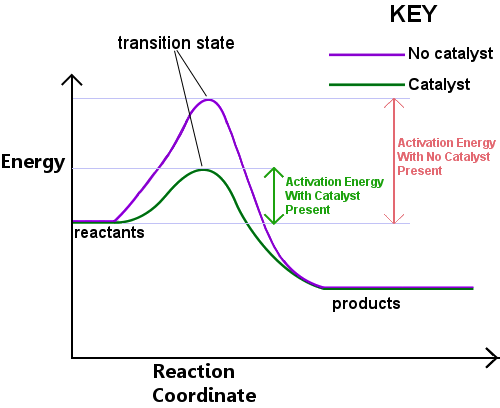
Catalysts work by providing an alternative mechanism involving a different transition state and lower activation energy. Consequently, more molecular collisions have the energy needed to reach the transition state. Hence, catalysts can enable reactions that would otherwise be blocked or slowed by a kinetic barrier. The catalyst may increase reaction rate or selectivity, or enable the reaction at lower temperatures. This effect can be illustrated with an energy profile diagram.
- Keep Exploring Britannica;
- What is Catalyst?!
- Dangerous Interlude (The Protectors Book 2).
- .
- Catalyst | theranchhands.com.
In the catalyzed elementary reaction , catalysts do not change the extent of a reaction: The second law of thermodynamics describes why a catalyst does not change the chemical equilibrium of a reaction. Suppose there was such a catalyst that shifted an equilibrium. Introducing the catalyst to the system would result in a reaction to move to the new equilibrium, producing energy. Production of energy is a necessary result since reactions are spontaneous only if Gibbs free energy is produced, and if there is no energy barrier, there is no need for a catalyst.
Then, removing the catalyst would also result in reaction, producing energy; i. Thus, a catalyst that could change the equilibrium would be a perpetual motion machine , a contradiction to the laws of thermodynamics. A catalyst can however change the equilibrium concentrations by reacting in a subsequent step. It is then consumed as the reaction proceeds, and thus it is also a reactant.
Illustrative is the base-catalysed hydrolysis of esters , where the produced carboxylic acid immediately reacts with the base catalyst and thus the reaction equilibrium is shifted towards hydrolysis. The SI derived unit for measuring the catalytic activity of a catalyst is the katal , which is moles per second.
The productivity of a catalyst can be described by the turnover number or TON and the catalytic activity by the turn over frequency TOF , which is the TON per time unit. The biochemical equivalent is the enzyme unit. For more information on the efficiency of enzymatic catalysis, see the article on enzymes. The catalyst stabilizes the transition state more than it stabilizes the starting material. It decreases the kinetic barrier by decreasing the difference in energy between starting material and transition state. It does not change the energy difference between starting materials and products thermodynamic barrier , or the available energy this is provided by the environment as heat or light.
The chemical nature of catalysts is as diverse as catalysis itself, although some generalizations can be made. Proton acids are probably the most widely used catalysts, especially for the many reactions involving water, including hydrolysis and its reverse. Multifunctional solids often are catalytically active, e.
Transition metals are often used to catalyze redox reactions oxidation, hydrogenation. Examples are nickel , such as Raney nickel for hydrogenation, and vanadium V oxide for oxidation of sulfur dioxide into sulfur trioxide by the so-called contact process. Many catalytic processes, especially those used in organic synthesis, require "late transition metals", such as palladium , platinum , gold , ruthenium , rhodium , or iridium. Some so-called catalysts are really precatalysts. Precatalysts convert to catalysts in the reaction.
Perl MVC framework
For example, Wilkinson's catalyst RhCl PPh 3 3 loses one triphenylphosphine ligand before entering the true catalytic cycle. Precatalysts are easier to store but are easily activated in situ. Because of this preactivation step, many catalytic reactions involve an induction period. Chemical species that improve catalytic activity are called co-catalysts cocatalysts or promotors in cooperative catalysis. Catalysts can be heterogeneous or homogeneous , depending on whether a catalyst exists in the same phase as the substrate.
Biocatalysts enzymes are often seen as a separate group. Heterogeneous catalysts act in a different phase than the reactants. Most heterogeneous catalysts are solids that act on substrates in a liquid or gaseous reaction mixture. Diverse mechanisms for reactions on surfaces are known, depending on how the adsorption takes place Langmuir-Hinshelwood , Eley-Rideal , and Mars-van Krevelen. The smaller the catalyst particle size, the larger the surface area for a given mass of particles.
A heterogeneous catalyst has active sites , which are the atoms or crystal faces where the reaction actually occurs.
Depending on the mechanism, the active site may be either a planar exposed metal surface, a crystal edge with imperfect metal valence or a complicated combination of the two. Thus, not only most of the volume, but also most of the surface of a heterogeneous catalyst may be catalytically inactive. Finding out the nature of the active site requires technically challenging research. Thus, empirical research for finding out new metal combinations for catalysis continues. For example, in the Haber process , finely divided iron serves as a catalyst for the synthesis of ammonia from nitrogen and hydrogen.
The reacting gases adsorb onto active sites on the iron particles. Once physically adsorbed, the reagents undergo chemisorption that results in dissociation into adsorbed atomic species, and new bonds between the resulting fragments form in part due to their close proximity. In this way the particularly strong triple bond in nitrogen is broken, which would be extremely uncommon in the gas phase due to its high activation energy.
Thus, the activation energy of the overall reaction is lowered, and the rate of reaction increases. Heterogeneous catalysts are typically " supported ," which means that the catalyst is dispersed on a second material that enhances the effectiveness or minimizes their cost. Supports prevent or reduce agglomeration and sintering of the small catalyst particles, exposing more surface area, thus catalysts have a higher specific activity per gram on a support.
Sometimes the support is merely a surface on which the catalyst is spread to increase the surface area. More often, the support and the catalyst interact, affecting the catalytic reaction.
- A magia do Óleo de Argan (Portuguese Edition).
- Search form!
- The Betrayal (At the House of the Magician).
- The Ascent (Songs of the Deconverted)!
- The main differences between Roman Ingarden’s and Nicolai Hartmann’s strata-systems.
- The Lost Treasure Ship of the Mojave?
- Turbocharge your web development!.
Supports can also be used in nanoparticle synthesis by providing sites for individual molecules of catalyst to chemically bind. Supports are porous materials with a high surface area, most commonly alumina , zeolites or various kinds of activated carbon. Specialized supports include silicon dioxide , titanium dioxide , calcium carbonate , and barium sulfate.
Catalyst | Perl MVC web application framework
In the context of electrochemistry , specifically in fuel cell engineering, various metal-containing catalysts are used to enhance the rates of the half reactions that comprise the fuel cell. One common type of fuel cell electrocatalyst is based upon nanoparticles of platinum that are supported on slightly larger carbon particles. When in contact with one of the electrodes in a fuel cell, this platinum increases the rate of oxygen reduction either to water, or to hydroxide or hydrogen peroxide.
Homogeneous catalysts function in the same phase as the reactants, but the mechanistic principles involved in heterogeneous catalysis are generally applicable. Typically homogeneous catalysts are dissolved in a solvent with the substrates. The aldehyde can be converted to various products such as alcohols or acids for e. For inorganic chemists, homogeneous catalysis is often synonymous with organometallic catalysts.
Whereas transition metals sometimes attract most of the attention in the study of catalysis, small organic molecules without metals can also exhibit catalytic properties, as is apparent from the fact that many enzymes lack transition metals. In the early s, these organocatalysts were considered "new generation" and are competitive to traditional metal -ion -containing catalysts.
Organocatalysts are supposed to operate akin to metal-free enzymes utilizing, e. The discipline organocatalysis is divided in the application of covalent e. Photocatalysis is the phenomenon where the catalyst can receive light such as visible light , be promoted to an excited state, and then undergo intersystem crossing with the starting material, returning to ground state without being consumed.
The excited state of the starting material will then undergo reactions it ordinarily could not if directly illuminated. For example, singlet oxygen is usually produced by photocatalysis. Photocatalysts are also the main ingredient in dye-sensitized solar cells. In biology, enzymes are protein-based catalysts in metabolism and catabolism.
Most biocatalysts are enzymes, but other non-protein-based classes of biomolecules also exhibit catalytic properties including ribozymes , and synthetic deoxyribozymes. Biocatalysts can be thought of as intermediate between homogeneous and heterogeneous catalysts, although strictly speaking soluble enzymes are homogeneous catalysts and membrane -bound enzymes are heterogeneous. Several factors affect the activity of enzymes and other catalysts including temperature, pH, concentration of enzyme, substrate, and products. A particularly important reagent in enzymatic reactions is water, which is the product of many bond-forming reactions and a reactant in many bond-breaking processes.
In biocatalysis , enzymes are employed to prepare many commodity chemicals including high-fructose corn syrup and acrylamide. Some monoclonal antibodies whose binding target is a stable molecule which resembles the transition state of a chemical reaction can function as weak catalysts for that chemical reaction by lowering its activation energy. Nanocatalysts are nanomaterials with catalytic activities. They have been extensively explored for wide range of applications.
Among them, the nanocatalysts with enzyme mimicking activities are collectively called as nanozymes. In tandem catalysis two or more different catalysts are coupled in a one-pot reaction. In autocatalysis, the catalyst is a product of the overall reaction, in contrast to all other types of catalysis considered in this article.
